Monday Poster Session
Category: IBD
P2628 - Real-World Effectiveness of Ozanimod for Ulcerative Colitis in Patients With Prior Advanced Therapy Exposure: A Multicenter Study
Monday, October 28, 2024
10:30 AM - 4:00 PM ET
Location: Exhibit Hall E
- SL
Stephen Lieto, MD
Mount Sinai Hospital
New York, New York
Presenting Author(s)
Nicholas Scalzo, MD1, Stephen Lieto, MD2, Katherine (Lulu) Huang, 3, Abdul Khan, 4, Anthony Xu, MD5, Joel Pekow, MD6, Palak Rajauria, BA, MS7, Malek Ayoub, MD8, Amanda M. Johnson, MD9, Andres Yarur, MD4, Anish Patel, DO10, David Dulaney, MD11, Nikhil Seth, MD12, Shrinivas Bishu, MD13, Richa Shukla, MD5, Anjali Jain, PhD14, Uyen Sokol, PharmD15, David T.. Rubin, MD, FACG6, Bruce E.. Sands, MD, FACG7, Parakkal Deepak, MBBS, MS16, Ryan C. Ungaro, MD7
1The Mount Sinai Hospital, New York, NY; 2Mount Sinai Hospital, New York, NY; 3Washington University, St. Louis, MO; 4Cedars-Sinai Medical Center, Los Angeles, CA; 5Baylor College of Medicine, Houston, TX; 6University of Chicago Medicine, Inflammatory Bowel Disease Center, Chicago, IL; 7Icahn School of Medicine at Mount Sinai, New York, NY; 8Washington University School of Medicine in St. Louis, St. Louis, MO; 9Mayo Clinic, Rochester, MN; 10Brooke Army Medical Center, Fort Sam Houston, TX; 11Brooke Army Medical Center, San Antonio, TX; 12Brooke Army Medical Center, Houston, TX; 13University of Michigan, Ann Arbor, MI; 14Bristol Myers Squibb, Princeton, NJ; 15Bristol Myers Squibb, New York, NY; 16Washington University in St. Louis, St. Louis, MO
Introduction: Ozanimod (OZA), an oral sphinosine-1-phosphate receptor modulator, has demonstrated efficacy and safety in patients with moderate to severe Ulcerative Colitis (UC) in clinical trials. We aimed to describe the real-world effectiveness of OZA based on prior advanced therapy (AT) exposure.
Methods: We conducted a retrospective analysis of data from the multicenter REal world Biologics and small mOlecules OuTcomes in IBD (REBOOT-IBD) consortium, which includes 10 tertiary centers in the United States. Adult patients with UC, newly started on OZA, were evaluated. Inclusion criteria were age > 17 years, established UC diagnosis, and at least one follow-up encounter following OZA initiation. Data was abstracted from patient electronic medical records using standardized case report forms. Primary outcome was clinical remission (CR) at 12 weeks, defined as partial Mayo score (PMS) < 2. Clinical remission was further analyzed by prior AT exposure. AT exposure was defined as any prior use of anti-tumor necrosis factor (TNF) agents (infliximab, adalimumab, and golimumab), JAK inhibitors (tofacitinib and upadacitinib), vedolizumab and/or ustekinumab. Descriptive statistics and bivariate comparisons were performed.
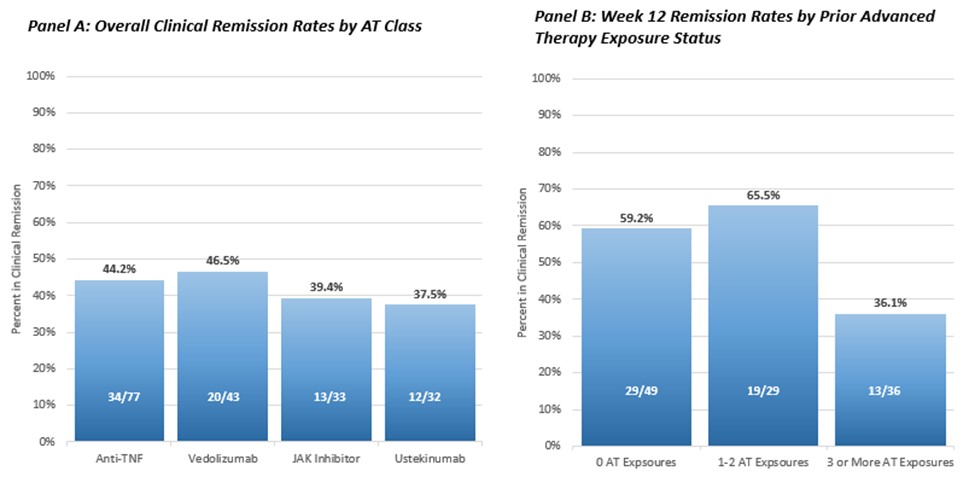
Results: A total of 146 patients were included in the analysis. Baseline demographics and clinical characteristics are provided in Table 1. OZA demonstrated similar effectiveness at inducing CR in AT-naïve patients, [59.2% (29/49)] and in patients with 1-2 prior AT exposures [65.6%(19/29)] (Figure 1, Panel B). We observed that 36.1% (13/36) of patients with 3 or more AT exposures were in CR at week 12. Week 12 CR rates stratified by prior AT exposure class are presented in Figure 1, Panel A). Among 5 patients exposed to vedolizumab only prior to OZA, 80% (4/5) were in CR and among 7 patients exposed to anti-TNF only, 86% (6/7) were in CR.
Discussion: Ozanimod is effective at inducing CR in both AT-naïve patients and those with prior AT exposures in a real-world setting. Remission rates are lower once patients have had 3 or more prior AT exposures.
Note: The table for this abstract can be viewed in the ePoster Gallery section of the ACG 2024 ePoster Site or in The American Journal of Gastroenterology's abstract supplement issue, both of which will be available starting October 27, 2024.
Disclosures:
Nicholas Scalzo, MD1, Stephen Lieto, MD2, Katherine (Lulu) Huang, 3, Abdul Khan, 4, Anthony Xu, MD5, Joel Pekow, MD6, Palak Rajauria, BA, MS7, Malek Ayoub, MD8, Amanda M. Johnson, MD9, Andres Yarur, MD4, Anish Patel, DO10, David Dulaney, MD11, Nikhil Seth, MD12, Shrinivas Bishu, MD13, Richa Shukla, MD5, Anjali Jain, PhD14, Uyen Sokol, PharmD15, David T.. Rubin, MD, FACG6, Bruce E.. Sands, MD, FACG7, Parakkal Deepak, MBBS, MS16, Ryan C. Ungaro, MD7. P2628 - Real-World Effectiveness of Ozanimod for Ulcerative Colitis in Patients With Prior Advanced Therapy Exposure: A Multicenter Study, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
1The Mount Sinai Hospital, New York, NY; 2Mount Sinai Hospital, New York, NY; 3Washington University, St. Louis, MO; 4Cedars-Sinai Medical Center, Los Angeles, CA; 5Baylor College of Medicine, Houston, TX; 6University of Chicago Medicine, Inflammatory Bowel Disease Center, Chicago, IL; 7Icahn School of Medicine at Mount Sinai, New York, NY; 8Washington University School of Medicine in St. Louis, St. Louis, MO; 9Mayo Clinic, Rochester, MN; 10Brooke Army Medical Center, Fort Sam Houston, TX; 11Brooke Army Medical Center, San Antonio, TX; 12Brooke Army Medical Center, Houston, TX; 13University of Michigan, Ann Arbor, MI; 14Bristol Myers Squibb, Princeton, NJ; 15Bristol Myers Squibb, New York, NY; 16Washington University in St. Louis, St. Louis, MO
Introduction: Ozanimod (OZA), an oral sphinosine-1-phosphate receptor modulator, has demonstrated efficacy and safety in patients with moderate to severe Ulcerative Colitis (UC) in clinical trials. We aimed to describe the real-world effectiveness of OZA based on prior advanced therapy (AT) exposure.
Methods: We conducted a retrospective analysis of data from the multicenter REal world Biologics and small mOlecules OuTcomes in IBD (REBOOT-IBD) consortium, which includes 10 tertiary centers in the United States. Adult patients with UC, newly started on OZA, were evaluated. Inclusion criteria were age > 17 years, established UC diagnosis, and at least one follow-up encounter following OZA initiation. Data was abstracted from patient electronic medical records using standardized case report forms. Primary outcome was clinical remission (CR) at 12 weeks, defined as partial Mayo score (PMS) < 2. Clinical remission was further analyzed by prior AT exposure. AT exposure was defined as any prior use of anti-tumor necrosis factor (TNF) agents (infliximab, adalimumab, and golimumab), JAK inhibitors (tofacitinib and upadacitinib), vedolizumab and/or ustekinumab. Descriptive statistics and bivariate comparisons were performed.
Results: A total of 146 patients were included in the analysis. Baseline demographics and clinical characteristics are provided in Table 1. OZA demonstrated similar effectiveness at inducing CR in AT-naïve patients, [59.2% (29/49)] and in patients with 1-2 prior AT exposures [65.6%(19/29)] (Figure 1, Panel B). We observed that 36.1% (13/36) of patients with 3 or more AT exposures were in CR at week 12. Week 12 CR rates stratified by prior AT exposure class are presented in Figure 1, Panel A). Among 5 patients exposed to vedolizumab only prior to OZA, 80% (4/5) were in CR and among 7 patients exposed to anti-TNF only, 86% (6/7) were in CR.
Discussion: Ozanimod is effective at inducing CR in both AT-naïve patients and those with prior AT exposures in a real-world setting. Remission rates are lower once patients have had 3 or more prior AT exposures.

Figure: Figure 1: Week 12 Remission Rates by prior AT Exposure
Note: The table for this abstract can be viewed in the ePoster Gallery section of the ACG 2024 ePoster Site or in The American Journal of Gastroenterology's abstract supplement issue, both of which will be available starting October 27, 2024.
Disclosures:
Nicholas Scalzo indicated no relevant financial relationships.
Stephen Lieto indicated no relevant financial relationships.
Katherine (Lulu) Huang indicated no relevant financial relationships.
Abdul Khan indicated no relevant financial relationships.
Anthony Xu indicated no relevant financial relationships.
Joel Pekow: Abbot Labs – Stock-publicly held company(excluding mutual/index funds). CVS health – Consultant. Eli Lilly – Stock-publicly held company(excluding mutual/index funds). Johnson and Johnson – Stock-publicly held company(excluding mutual/index funds). Pfizer – Stock-publicly held company(excluding mutual/index funds). Takeda – Grant/Research Support.
Palak Rajauria indicated no relevant financial relationships.
Malek Ayoub indicated no relevant financial relationships.
Amanda Johnson indicated no relevant financial relationships.
Andres Yarur: AbbVie – Consultant, served on clinical trial steering committee. Abivax – Advisory Committee/Board Member, Consultant. Arena – Consultant, served on clinical trial steering committee. Boehringer Ingelheim – Advisory Committee/Board Member, Consultant. Bristol Myers Squibb – Consultant, served on clinical trial steering committee. Celltrion – Consultant, served on clinical trial steering committee. Johnson and Johnson – Advisory Committee/Board Member, Consultant. Pfizer – Consultant, served on clinical trial steering committee. Takeda – Consultant, served on clinical trial steering committee.
Anish Patel: Abbvie – Consultant. BMS – Speakers Bureau. Eli Lily – Speakers Bureau. Janssen – Speakers Bureau. Pfizer – Speakers Bureau. Phathom Pharmaceuticals – Speakers Bureau. Takeda – Speakers Bureau.
David Dulaney: Regeneron – Speaker. Takeda – Speaker.
Nikhil Seth indicated no relevant financial relationships.
Shrinivas Bishu: Abbvie – Consultant. Bristol Myers Squibb – Consultant. Crohn's Colitis Foundation – Grant/Research Support. Janssen – Consultant. NIH – Grant/Research Support. Takeda – Consultant.
Richa Shukla: Abbvie – Speakers Bureau.
Anjali Jain: Bristol Myers Squibb – Employee.
Uyen Sokol: Bristol Myers Squibb – Employee.
David Rubin: AbbVie – Consultant. AltruBio – Consultant. Apex – Consultant. Avalo Therapeutics – Consultant. Bausch Health – Consultant. Bristol Myers Squibb – Consultant. Buhlmann Diagnostics Corp – Consultant. Celgene – Consultant. ClostraBio – Consultant. Connect BioPharma – Consultant. Cornerstones Health – Board of Directors. Crohn's & Colitis Foundation – Board of Trustees. Douglas Therapeutics – Consultant. Eli Lilly – Consultant. InDex Pharmaceuticals – Consultant. Intouch Group – Consultant. Iterative Health – Consultant. Janssen Pharmaceuticals – Consultant. Odyssey Thera – Consultant. Pfizer – Consultant. Prometheus Biosciences – Consultant. Samsung Neurologica – Consultant. Takeda – Consultant, Grant/Research Support.
Bruce Sands: AbbVie – Consultant. Abivax – Consultant, Speakers Bureau. Adiso Therapeutics – Consultant. Agomab – Consultant. Alimentiv – Consultant. Amgen – Consultant. AnaptysBio – Consultant. Arena Pharmaceuticals – Consultant. Artugen Therapeutics – Consultant. AstraZeneca – Consultant. Biolojic Design – Consultant. Biora Therapeutics – Consultant. Boehringer Ingelheim – Consultant. Boston Pharmaceuticals – Consultant. Bristol Myers Squibb – Consultant, Grant/Research Support, Other support, Speakers Bureau. Calibr – Consultant. Celgene – Consultant. Celltrion – Consultant. ClostraBio – Consultant. Enthera – Consultant. Envied Biosciences – Consultant. Equilium – Consultant. Evommune – Consultant. Ferring – Consultant. Fiat – Consultant. Fresenius Kabi – Consultant. Galapagos – Consultant. Genentech (Roche) – Consultant. Gilead Sciences – Consultant. Glaxo SmithKline – Consultant. Gossamer Bio – Consultant. Imhotex – Consultant. Index Pharmaceuticals – Consultant. Innovation Pharmaceuticals – Consultant. Inotrem – Consultant. Janssen – Consultant, Grant/Research Support, Other support, Speakers Bureau. Kaleido – Consultant. Kallyope – Consultant. Lilly – Consultant, other support, Speakers Bureau. Merck & Co., Inc., Rahway, NJ, USA – Consultant. Microba – Consultant. Mobius Care – Consultant. Morphic Therapeutics – Consultant. MRM Health – Consultant. Nexus Therapeutics – Consultant. Nimbus Discovery – Consultant. Odyssey Therapeutics – Consultant. Pfizer Inc – Consultant, Grant/Research Support, Other support, Speakers Bureau. Progenity – Consultant. Prometheus Biosciences – Consultant. Prometheus Laboratories – Consultant. Protagonist Therapeutics – Consultant. Q32 Bio – Consultant. Rasayana Therapeutics – Consultant. Recludix Therapeutics – Consultant. Reistone Biopharma – Consultant. Sanofi – Consultant. Spyre Therapeutics – Consultant. Sun Pharma – Consultant. Surrozen – Consultant. Takeda – Consultant, Grant/Research Support, Other support, Speakers Bureau. Target RWE – Consultant. Teva – Consultant. Theravance Biopharma – Consultant, Grant/Research Support. TLL Pharmaceutical – Consultant. Tr1X – Consultant. Union Therapeutics – Consultant. Ventyx Biopharma – Consultant, Stock Options, Stock-publicly held company(excluding mutual/index funds).
Parakkal Deepak: AbbVie – Consultant, Grant/Research Support. Alimentiv – Grant/Research Support. Arena Pharmaceuticals – Grant/Research Support. Boehringer Ingelheim – Grant/Research Support. Bristol Myers Squibb-Celgene – Advisory Committee/Board Member, Grant/Research Support. CorEvitas LLC – Consultant. Janssen – Grant/Research Support. Pfizer – Grant/Research Support. Prometheus Biosciences – Grant/Research Support. Roche/Genentech – Advisory Committee/Board Member. Scipher Medicine – Grant/Research Support. Takeda – Grant/Research Support.
Ryan C. Ungaro: AbbVie – Advisory Committee/Board Member, Grant/Research Support. Boehringer Ingelheim – Grant/Research Support. Bristol Myers Squibb – Advisory Committee/Board Member. Celltrion – Advisory Committee/Board Member, Consultant. Eli Lilly – Grant/Research Support. Inotrem – Advisory Committee/Board Member, Consultant. Janssen – Advisory Committee/Board Member. Pfizer – Advisory Committee/Board Member, Grant/Research Support. Prometheus Labratories – Grant/Research Support. Roivant – Advisory Committee/Board Member, Consultant. Takeda – Advisory Committee/Board Member.
Nicholas Scalzo, MD1, Stephen Lieto, MD2, Katherine (Lulu) Huang, 3, Abdul Khan, 4, Anthony Xu, MD5, Joel Pekow, MD6, Palak Rajauria, BA, MS7, Malek Ayoub, MD8, Amanda M. Johnson, MD9, Andres Yarur, MD4, Anish Patel, DO10, David Dulaney, MD11, Nikhil Seth, MD12, Shrinivas Bishu, MD13, Richa Shukla, MD5, Anjali Jain, PhD14, Uyen Sokol, PharmD15, David T.. Rubin, MD, FACG6, Bruce E.. Sands, MD, FACG7, Parakkal Deepak, MBBS, MS16, Ryan C. Ungaro, MD7. P2628 - Real-World Effectiveness of Ozanimod for Ulcerative Colitis in Patients With Prior Advanced Therapy Exposure: A Multicenter Study, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
