Monday Poster Session
Category: IBD
P2665 - Biologic Therapy Is Not Associated With Increased Risk of Cancer or Infection in Elderly Patients With Inflammatory Bowel Disease
Monday, October 28, 2024
10:30 AM - 4:00 PM ET
Location: Exhibit Hall E
Has Audio
- JC
Jennifer Chan, MD
Kaiser Permanente
Redwood City, CA
Presenting Author(s)
Jennifer Chan, MD1, Abhik Roy, MD2, Elijah wade, MPH3, Lue-Yen Tucker, BA4, Fernando Velayos, MD, MPH5
1Kaiser Permanente, Redwood City, CA; 2Kaiser Permanente, San Leandro, CA; 3Kaiser Permanente Northern California, Oakland, CA; 4Kaiser Permanente, Pleasanton, CA; 5Kaiser Permanente, San Francisco, CA
Introduction: Like younger patients, older patients with inflammatory bowel disease (IBD) may require treatment with immunosuppressive medications for disease management. Undertreatment may occur given the paucity of data on the safety of biologic therapies in the elderly population, specifically regarding infection and cancer risk. We aimed to evaluate the association between biologic therapy (overall and by type) and serious infection or cancer in these patients.
Methods: We conducted a retrospective, cohort study of patients 60 and older with a diagnosis of IBD between 2013-2020 at a large, integrated healthcare delivery system caring for more than 4.5 million persons who are broadly representative of the local and statewide population.
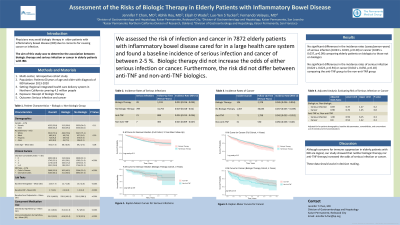
Results: The final analytical cohort included 7872 patients: 54% female, 75% Caucasian, mean age 69 years, 66% having ulcerative colitis and 34% having Crohn’s disease. 616 patients (7.8%) were treated with biologic agents: 433 (70.3%) were on anti-tumor necrosis factor (TNF) drugs while the remainder were on non-anti-TNF agents. Comparing patients in the biologic group to those in the non-biologic group, there were no significant differences in the incidence rates (cases/person-years) of serious infection (0.023 vs 0.019, p=0.30) or cancer (0.045 vs 0.037, p=0.09). When comparing the anti-TNF to non-anti-TNF groups, there were no significant differences in the incidence rates of serious infection (0.024 vs 0.023, p=0.94) or cancer (0.042 vs 0.056, p=0.18). In multivariable Cox regression adjusting for patient demographics, baseline lab parameters, comorbidities, and concomitant use of steroids and immunomodulators, there remained no differences (biologic vs non-biologic) in the risk of serious infection (adjusted hazard ratio [aHR] 0.69, 95% Confidence Interval [CI] 0.37-1.27, p=0.2) or cancer (aHR 0.98, 95%CI 0.76-1.25, p=0.8). Subset analyses (anti-TNF vs non-anti-TNF groups) showed similar results (serious infection: aHR 1.90, 95%CI 0.59-6.15, p=0.3; cancer: aHR 0.8, 95%CI 0.52-1.22, p=0.3).
Discussion: The overall incidence of serious infection and cancer in elderly IBD patients is between 2-5% each year. The risk of serious infection or cancer did not differ between those on biologics compared to those not on biologics, nor did it differ between those on anti-TNF compared to those on non-anti-TNF biologics. These data can help inform treatment decisions in this vulnerable population.
Disclosures:
Jennifer Chan, MD1, Abhik Roy, MD2, Elijah wade, MPH3, Lue-Yen Tucker, BA4, Fernando Velayos, MD, MPH5. P2665 - Biologic Therapy Is Not Associated With Increased Risk of Cancer or Infection in Elderly Patients With Inflammatory Bowel Disease, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
1Kaiser Permanente, Redwood City, CA; 2Kaiser Permanente, San Leandro, CA; 3Kaiser Permanente Northern California, Oakland, CA; 4Kaiser Permanente, Pleasanton, CA; 5Kaiser Permanente, San Francisco, CA
Introduction: Like younger patients, older patients with inflammatory bowel disease (IBD) may require treatment with immunosuppressive medications for disease management. Undertreatment may occur given the paucity of data on the safety of biologic therapies in the elderly population, specifically regarding infection and cancer risk. We aimed to evaluate the association between biologic therapy (overall and by type) and serious infection or cancer in these patients.
Methods: We conducted a retrospective, cohort study of patients 60 and older with a diagnosis of IBD between 2013-2020 at a large, integrated healthcare delivery system caring for more than 4.5 million persons who are broadly representative of the local and statewide population.
Results: The final analytical cohort included 7872 patients: 54% female, 75% Caucasian, mean age 69 years, 66% having ulcerative colitis and 34% having Crohn’s disease. 616 patients (7.8%) were treated with biologic agents: 433 (70.3%) were on anti-tumor necrosis factor (TNF) drugs while the remainder were on non-anti-TNF agents. Comparing patients in the biologic group to those in the non-biologic group, there were no significant differences in the incidence rates (cases/person-years) of serious infection (0.023 vs 0.019, p=0.30) or cancer (0.045 vs 0.037, p=0.09). When comparing the anti-TNF to non-anti-TNF groups, there were no significant differences in the incidence rates of serious infection (0.024 vs 0.023, p=0.94) or cancer (0.042 vs 0.056, p=0.18). In multivariable Cox regression adjusting for patient demographics, baseline lab parameters, comorbidities, and concomitant use of steroids and immunomodulators, there remained no differences (biologic vs non-biologic) in the risk of serious infection (adjusted hazard ratio [aHR] 0.69, 95% Confidence Interval [CI] 0.37-1.27, p=0.2) or cancer (aHR 0.98, 95%CI 0.76-1.25, p=0.8). Subset analyses (anti-TNF vs non-anti-TNF groups) showed similar results (serious infection: aHR 1.90, 95%CI 0.59-6.15, p=0.3; cancer: aHR 0.8, 95%CI 0.52-1.22, p=0.3).
Discussion: The overall incidence of serious infection and cancer in elderly IBD patients is between 2-5% each year. The risk of serious infection or cancer did not differ between those on biologics compared to those not on biologics, nor did it differ between those on anti-TNF compared to those on non-anti-TNF biologics. These data can help inform treatment decisions in this vulnerable population.
Disclosures:
Jennifer Chan indicated no relevant financial relationships.
Abhik Roy indicated no relevant financial relationships.
Elijah wade indicated no relevant financial relationships.
Lue-Yen Tucker indicated no relevant financial relationships.
Fernando Velayos indicated no relevant financial relationships.
Jennifer Chan, MD1, Abhik Roy, MD2, Elijah wade, MPH3, Lue-Yen Tucker, BA4, Fernando Velayos, MD, MPH5. P2665 - Biologic Therapy Is Not Associated With Increased Risk of Cancer or Infection in Elderly Patients With Inflammatory Bowel Disease, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
