Monday Poster Session
Category: IBD
P2671 - Colonic Bioactivation and Potent TNFa Inhibition of PALI-2108 in Human ex-vivo Studies: A Promising PDE4 Inhibitor Prodrug for the Oral Treatment of Ulcerative Colitis
Monday, October 28, 2024
10:30 AM - 4:00 PM ET
Location: Exhibit Hall E
.jpg)
Mitchell Jones, MD, PhD
Palisade Bio
Carlsbad, CA
Presenting Author(s)
Joie Bernard, PhD.1, Florian Rieder, MD2, Christophe Mellon, PhD.3, Mitchell Jones, MD, PhD1
1Palisade Bio, Carlsbad, CA; 2Digestive Diseases and Surgery Institute; Lerner Research Institute, Program for Global Translational Inflammatory Bowel Diseases; Cleveland Clinic Foundation, Cleveland, OH; 3Giiant Pharma, Carlsbad, CA
Introduction: PDE4 is a key enzyme in cAMP hydrolysis. Inhibition elevates cAMP, downregulates inflammatory cytokines, and prevents local infiltration of inflammatory cells. Approved PDE4 inhibitors include roflumilast for COPD and apremilast for psoriasis. Despite the development of subtype specific PDE4 inhibitors, oral administration and systemic distribution has resulted in CNS toxicity (headaches, nausea or vomiting) leading to discontinuation of therapy and limiting potential efficacy. Targeted and better-tolerated oral PDE4 inhibitors remain an unmet need in IBD.
Methods: We investigated PALI-2108, an orally delivered, intestinally activated PDE4 inhibitor prodrug in two human translational studies. PALI-2108 minimizes systemic exposure until cleaved by colonic bacterium, reducing potential CNS toxicity. We first evaluated prodrug conversion in human stool samples using LC-MS to detect prodrug-to-drug conversion over time in samples from each individual donor. Second, the effect of the active PDE4 inhibitor (PALI-0008) on lipopolysaccharide (LPS)-induced TNFα production was evaluated using an ex-vivo peripheral whole blood assay.
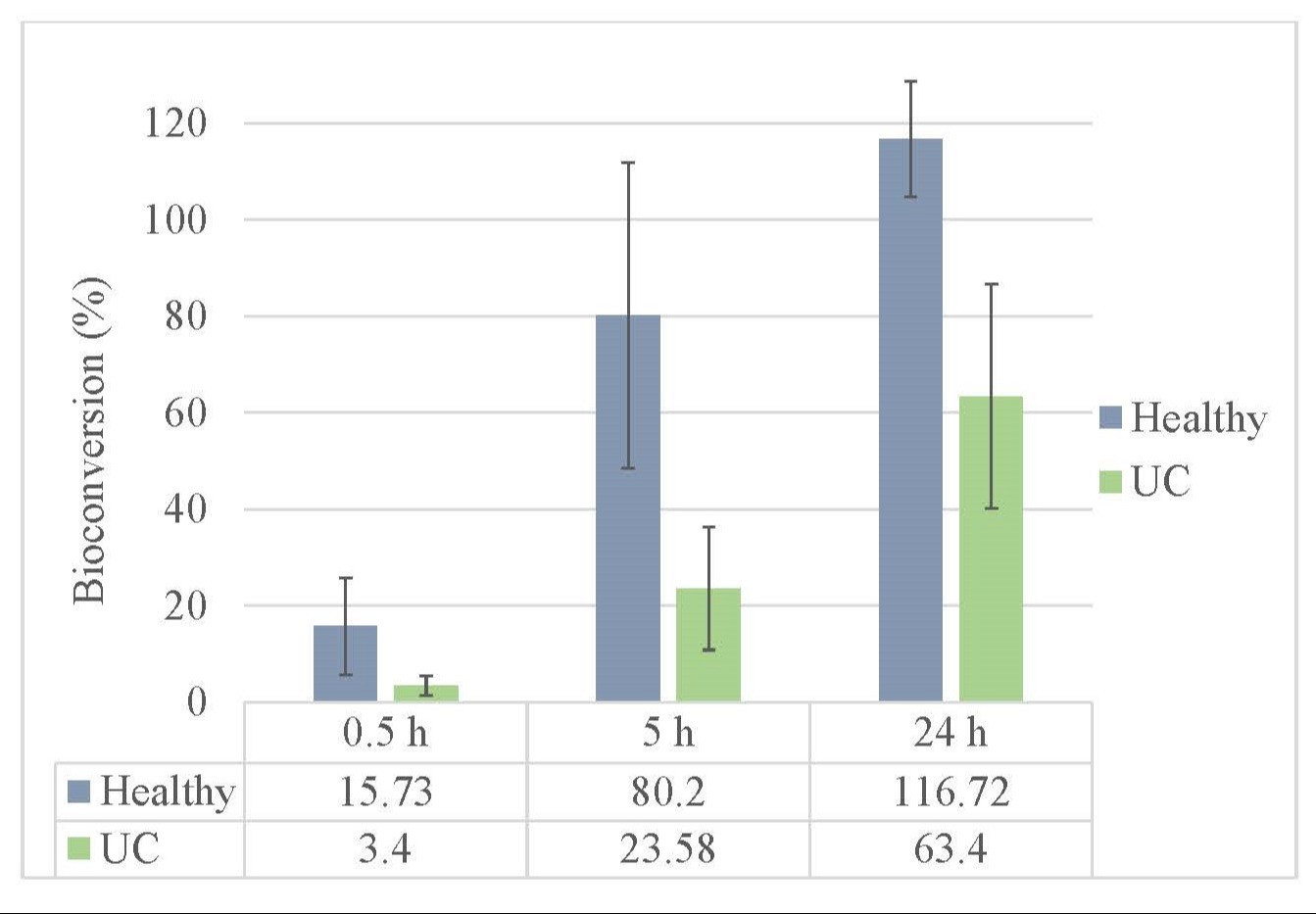
Results: We demonstrated effective intestinal hydrolysis of PALI-2108 prodrug into PALI-0008 using routine stool samples from 6 healthy patients and 6 patients with UC. PALI-2108 was spiked into stool homogenate (100 μM) and homogenate was subsequently incubated for 24 hours. Concentrations were measured and the prodrug-to-drug conversion percentage at 24 hours was a mean of 90.1% across samples. All subjects had conversion of greater than 30%. Conversion increased steadily over time beginning at 30 mins in normal healthy as well as UC patients stool samples. Second, pre-treatment of human whole blood from 12 healthy human donors with PALI-0008 (7-point titrations using 3-fold serial dilutions starting at 4 μM, in duplicate) followed by LPS challenge (1 μg/ml) for 24 hours, reduced the LPS induced TNFα production compared to non-pretreated samples. The IC50 values for PALI-0008 TNFα inhibition was 0.022 μM. Apremilast showed a 20-fold lower potency (IC50 of 0.41 μM) in the same assay.
Discussion: These studies demonstrate PALI-2108 converts into PALI-0008 ex-vivo in healthy human and UC patient stools and PALI-0008 show potent ability to suppress LPS induced TNFα in an ex-vivo whole blood assay. This data supports the potential of PALI-2108 as a gut targeted treatment for moderate-to-severe UC. Clinical development is ongoing with plans for first-in-human studies.
Note: The table for this abstract can be viewed in the ePoster Gallery section of the ACG 2024 ePoster Site or in The American Journal of Gastroenterology's abstract supplement issue, both of which will be available starting October 27, 2024.
Disclosures:
Joie Bernard, PhD.1, Florian Rieder, MD2, Christophe Mellon, PhD.3, Mitchell Jones, MD, PhD1. P2671 - Colonic Bioactivation and Potent TNFa Inhibition of PALI-2108 in Human ex-vivo Studies: A Promising PDE4 Inhibitor Prodrug for the Oral Treatment of Ulcerative Colitis, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
1Palisade Bio, Carlsbad, CA; 2Digestive Diseases and Surgery Institute; Lerner Research Institute, Program for Global Translational Inflammatory Bowel Diseases; Cleveland Clinic Foundation, Cleveland, OH; 3Giiant Pharma, Carlsbad, CA
Introduction: PDE4 is a key enzyme in cAMP hydrolysis. Inhibition elevates cAMP, downregulates inflammatory cytokines, and prevents local infiltration of inflammatory cells. Approved PDE4 inhibitors include roflumilast for COPD and apremilast for psoriasis. Despite the development of subtype specific PDE4 inhibitors, oral administration and systemic distribution has resulted in CNS toxicity (headaches, nausea or vomiting) leading to discontinuation of therapy and limiting potential efficacy. Targeted and better-tolerated oral PDE4 inhibitors remain an unmet need in IBD.
Methods: We investigated PALI-2108, an orally delivered, intestinally activated PDE4 inhibitor prodrug in two human translational studies. PALI-2108 minimizes systemic exposure until cleaved by colonic bacterium, reducing potential CNS toxicity. We first evaluated prodrug conversion in human stool samples using LC-MS to detect prodrug-to-drug conversion over time in samples from each individual donor. Second, the effect of the active PDE4 inhibitor (PALI-0008) on lipopolysaccharide (LPS)-induced TNFα production was evaluated using an ex-vivo peripheral whole blood assay.
Results: We demonstrated effective intestinal hydrolysis of PALI-2108 prodrug into PALI-0008 using routine stool samples from 6 healthy patients and 6 patients with UC. PALI-2108 was spiked into stool homogenate (100 μM) and homogenate was subsequently incubated for 24 hours. Concentrations were measured and the prodrug-to-drug conversion percentage at 24 hours was a mean of 90.1% across samples. All subjects had conversion of greater than 30%. Conversion increased steadily over time beginning at 30 mins in normal healthy as well as UC patients stool samples. Second, pre-treatment of human whole blood from 12 healthy human donors with PALI-0008 (7-point titrations using 3-fold serial dilutions starting at 4 μM, in duplicate) followed by LPS challenge (1 μg/ml) for 24 hours, reduced the LPS induced TNFα production compared to non-pretreated samples. The IC50 values for PALI-0008 TNFα inhibition was 0.022 μM. Apremilast showed a 20-fold lower potency (IC50 of 0.41 μM) in the same assay.
Discussion: These studies demonstrate PALI-2108 converts into PALI-0008 ex-vivo in healthy human and UC patient stools and PALI-0008 show potent ability to suppress LPS induced TNFα in an ex-vivo whole blood assay. This data supports the potential of PALI-2108 as a gut targeted treatment for moderate-to-severe UC. Clinical development is ongoing with plans for first-in-human studies.

Figure: Figure 1: Shows the percentage of PALI-2108 bioconversion to PALI-0008 as measured by PALI-0008 recovery via LC-MS/MS in stool samples of normal healthy and ulcerative colitis (UC) patient stool incubated over time.
Note: The table for this abstract can be viewed in the ePoster Gallery section of the ACG 2024 ePoster Site or in The American Journal of Gastroenterology's abstract supplement issue, both of which will be available starting October 27, 2024.
Disclosures:
Joie Bernard: Palisade Bio – Employee.
Florian Rieder: 89Bio – Consultant. AbbVie – Consultant, Grant/Research Support. Adiso – Consultant. Adnovate – Consultant. Agomab – Consultant. Allergan – Advisory Committee/Board Member, Consultant. Arena – Advisory Committee/Board Member, Consultant. AstraZeneca – Advisory Committee/Board Member, Consultant. Bausch & Lomb – Consultant. Boehringer Ingelheim – Advisory Committee/Board Member, Consultant, Grant/Research Support. CDISC – Consultant. Celgene/BMS – Advisory Committee/Board Member, Consultant, Grant/Research Support. Celltrion – Consultant. Celsius – Consultant. Cowen – Consultant. Eugit – Consultant. Ferring – Consultant. Galapagos – Consultant. Galmed – Consultant. Genentech – Advisory Committee/Board Member, Consultant. Gilead – Advisory Committee/Board Member, Consultant, Grant/Research Support. Gossamer – Consultant. Granite – Consultant. Guidepoint – Consultant. Helmsley – Consultant. Horizon Therapeutics – Consultant. Image Analysis Limited – Consultant. Index Pharma – Consultant. Jannsen – Consultant. Koutif – Consultant. Landos – Consultant. Mestag – Consultant. Metacrine – Consultant. Mirum – Consultant. Mopec – Consultant. Morphic – Consultant. Myka Labs – Consultant. Organovo – Consultant. Origo – Consultant. Palisade Bio – Consultant. Pfizer – Advisory Committee/Board Member, Consultant, Grant/Research Support. Pliant – Consultant. Prometheus Biosciences – Advisory Committee/Board Member, Consultant. Receptos – Consultant. RedX – Advisory Committee/Board Member, Consultant. Roche – Advisory Committee/Board Member, Consultant. Samsung – Advisory Committee/Board Member, Consultant. Sanofi – Consultant. Surmodics – Consultant. Surrozen – Consultant. Takeda – Advisory Committee/Board Member, Consultant, Grant/Research Support. Techlab – Consultant. Teva – Consultant. Theravance – Consultant. Thetis – Consultant. Trix Bio – Consultant. UCB – Advisory Committee/Board Member, Consultant, Grant/Research Support. Ysios – Consultant.
Christophe Mellon: Crohn's and Colitis Foundation – Grant/Research Support. Giiant Pharma – Stock-privately held company. Palisade Bio – Employee.
Mitchell Jones: Palisade Bio – Employee.
Joie Bernard, PhD.1, Florian Rieder, MD2, Christophe Mellon, PhD.3, Mitchell Jones, MD, PhD1. P2671 - Colonic Bioactivation and Potent TNFa Inhibition of PALI-2108 in Human ex-vivo Studies: A Promising PDE4 Inhibitor Prodrug for the Oral Treatment of Ulcerative Colitis, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
