Monday Poster Session
Category: IBD
P2673 - A Patient-Reported Outcome Measure Comprising the Stool Frequency and Abdominal Pain Items From the Crohn’s Disease Activity Index: Psychometric Evaluation in Adults With Crohn’s Disease
Monday, October 28, 2024
10:30 AM - 4:00 PM ET
Location: Exhibit Hall E
Has Audio
- JL
James Lewis, MD, MSCE
Center for Clinical Epidemiology and Biostatistics, University of Pennsylvania
Philadelphia, PA
Presenting Author(s)
James Lewis, MD, MSCE1, Aisha Vadhariya, 2, Sylvia Su, 2, Xian Zhou, 3, Frederick Durand, 2, Ariane Kawata, 4, Larissa Stassek, 4, Claudine Clucas, 4, Stefan Schreiber, MD5
1Center for Clinical Epidemiology and Biostatistics, University of Pennsylvania, Philadelphia, PA; 2Eli Lilly and Company, Indianapolis, IN; 3Syneos Health, Morrisville, NC; 4Evidera, Bethesda, MD; 5University Hospital, Kiel, Schleswig-Holstein, Germany
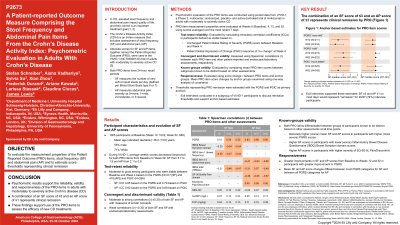
Introduction: Crohn’s Disease Activity Index (CDAI) is a disease activity measure with patient- and physician-reported items. Absolute scores from Stool Frequency (SF) and Abdominal Pain (AP) items of the CDAI (Patient-Reported Outcome [PRO]) have been used in the VIVID-1 study. This study assessed the measurement properties of SF and AP items and estimated remission thresholds.
Methods: Pooled Data from VIVID-1, a Phase 3 multicenter, randomized, placebo- and active-controlled study of mirikizumab in adults with moderately to severely active Crohn’s Disease (CD), were analyzed. Reliability, validity, and responsiveness of the PRO items were evaluated at Baseline (BL) and Week (W) 4, W12, and W52. Remission thresholds were assessed using anchor-based methods and exit interviews.
Results: Data from 1065 participants (mean age: 36.2±13.0 years, 55% males, 71% White) were analyzed. From BL to W52, average weekly scores decreased: SF, 5.7 to 1.9 and AP, 2.1 to 0.8. Both items demonstrated moderate-to-good test-retest reliability for participants who were stable between BL and W4 based on Patient Global Rating of Severity (PGRS) and Patient Global Impression of Change (PGIC) (intraclass correlation coefficient [ICC] 0.79 - 0.85 for SF and 0.69 - 0.82 for AP). As hypothesized, SF and AP demonstrated moderate to large correlations with the PGRS, PGIC and other related patient-reported outcome measures, demonstrating convergent validity; and both PRO items had weak correlations (|r| < 0.30) with endoscopic and laboratory assessments, demonstrating discriminant validity. The PRO items could discriminate between participant groups known to differ based on PGRS (for SF and AP), Inflammatory Bowel Disease Questionnaire Bowel Symptom domain score (for SF), and EQ-5D-5L Pain/Discomfort (for AP). PRO items could detect change, as score changes between BL and W12 and W52 differed statistically significantly among PGRS and PGIC groups. Anchor-based analyses combined with results from the exit interviews suggested that an SF score of ≤3 and an AP score of ≤1 would represent remission.
Discussion: The results support the reliability, construct-validity, and responsiveness of the PRO items when administered to adults with moderately to severely active CD. A combination of an SF score of ≤3 and AP score of ≤1 could represent clinical remission by PRO.
Disclosures:
James Lewis, MD, MSCE1, Aisha Vadhariya, 2, Sylvia Su, 2, Xian Zhou, 3, Frederick Durand, 2, Ariane Kawata, 4, Larissa Stassek, 4, Claudine Clucas, 4, Stefan Schreiber, MD5. P2673 - A Patient-Reported Outcome Measure Comprising the Stool Frequency and Abdominal Pain Items From the Crohn’s Disease Activity Index: Psychometric Evaluation in Adults With Crohn’s Disease, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
1Center for Clinical Epidemiology and Biostatistics, University of Pennsylvania, Philadelphia, PA; 2Eli Lilly and Company, Indianapolis, IN; 3Syneos Health, Morrisville, NC; 4Evidera, Bethesda, MD; 5University Hospital, Kiel, Schleswig-Holstein, Germany
Introduction: Crohn’s Disease Activity Index (CDAI) is a disease activity measure with patient- and physician-reported items. Absolute scores from Stool Frequency (SF) and Abdominal Pain (AP) items of the CDAI (Patient-Reported Outcome [PRO]) have been used in the VIVID-1 study. This study assessed the measurement properties of SF and AP items and estimated remission thresholds.
Methods: Pooled Data from VIVID-1, a Phase 3 multicenter, randomized, placebo- and active-controlled study of mirikizumab in adults with moderately to severely active Crohn’s Disease (CD), were analyzed. Reliability, validity, and responsiveness of the PRO items were evaluated at Baseline (BL) and Week (W) 4, W12, and W52. Remission thresholds were assessed using anchor-based methods and exit interviews.
Results: Data from 1065 participants (mean age: 36.2±13.0 years, 55% males, 71% White) were analyzed. From BL to W52, average weekly scores decreased: SF, 5.7 to 1.9 and AP, 2.1 to 0.8. Both items demonstrated moderate-to-good test-retest reliability for participants who were stable between BL and W4 based on Patient Global Rating of Severity (PGRS) and Patient Global Impression of Change (PGIC) (intraclass correlation coefficient [ICC] 0.79 - 0.85 for SF and 0.69 - 0.82 for AP). As hypothesized, SF and AP demonstrated moderate to large correlations with the PGRS, PGIC and other related patient-reported outcome measures, demonstrating convergent validity; and both PRO items had weak correlations (|r| < 0.30) with endoscopic and laboratory assessments, demonstrating discriminant validity. The PRO items could discriminate between participant groups known to differ based on PGRS (for SF and AP), Inflammatory Bowel Disease Questionnaire Bowel Symptom domain score (for SF), and EQ-5D-5L Pain/Discomfort (for AP). PRO items could detect change, as score changes between BL and W12 and W52 differed statistically significantly among PGRS and PGIC groups. Anchor-based analyses combined with results from the exit interviews suggested that an SF score of ≤3 and an AP score of ≤1 would represent remission.
Discussion: The results support the reliability, construct-validity, and responsiveness of the PRO items when administered to adults with moderately to severely active CD. A combination of an SF score of ≤3 and AP score of ≤1 could represent clinical remission by PRO.
Disclosures:
James Lewis: AbbVie – Personal fees, Non-financial support. Arena Parmaceuticals – Personal fees. Bridge Biotherapeutics – Personal fees. Bristol-Myers Squibb – Personal fees. Celgene – Personal fees. Eli Lilly and Company – Personal fees. Gilead – Personal fees. Janssen Pharmaceuticals – Grant/Research Support, Personal fees. Johnson & Johnson Consumer Inc – Personal fees. Merck – Personal fees. Nestle Health Science – Grant/Research Support, Personal fees. Pfizer – Grant/Research Support, Personal fees. Protagonist Therapeutics – Personal fees. Samsung Bioepis – Personal fees. Takeda Pharmaceuticals – Grant/Research Support, Personal fees. UCB – Personal fees.
Aisha Vadhariya: Eli Lilly and Company – Employee, Stock Options.
Sylvia Su: Eli Lilly and Company – Employee, Stock Options.
Xian Zhou: Syneos Health – Employee.
Frederick Durand: Eli Lilly and Company – Employee, Stock Options.
Ariane Kawata: Evidera – Employee.
Larissa Stassek: Evidera – Employee.
Claudine Clucas: Evidera – Employee.
Stefan Schreiber: AbbVie – Consultant, Personal fees, Speakers Bureau. Amgen – Personal fees. Arena Pharmaceuticals – Consultant, Personal fees, Speakers Bureau. Biogen – Consultant, Personal fees, Speakers Bureau. Bristol Myers Squibb – Consultant, Personal fees, Speakers Bureau. Celgene – Consultant, Personal fees, Speakers Bureau. Celltrion – Consultant, Personal fees, Speakers Bureau. Eli Lilly and Company – Personal fees. Falk – Consultant, Personal fees, Speakers Bureau. Ferring Pharmaceuticals – Personal fees. Fresenius – Consultant, Personal fees, Speakers Bureau. Galapagos – Personal fees. Gilead – Consultant, Personal fees. Hikma Pharmaceuticals – Advisory Committee/Board Member, Consultant. I-MAB – Consultant, Personal fees. Janssen – Consultant, Personal fees, Speakers Bureau. Morphic – Personal fees. MSD – Consultant, Personal fees, Speakers Bureau. Mylan – Consultant, Personal fees. Novartis – Personal fees. Pfizer Inc – Consultant, Personal fees, Speakers Bureau. Protagonist – Consultant, Personal fees. Provention Bio – Consultant, Personal fees. Roche – Personal fees. Sandoz/Hexal – Personal fees. Shire – Personal fees. Takeda – Consultant, Personal fees, Speakers Bureau. Theravance Biopharma – Consultant, Personal fees. Ventyx – Consultant, Personal fees.
James Lewis, MD, MSCE1, Aisha Vadhariya, 2, Sylvia Su, 2, Xian Zhou, 3, Frederick Durand, 2, Ariane Kawata, 4, Larissa Stassek, 4, Claudine Clucas, 4, Stefan Schreiber, MD5. P2673 - A Patient-Reported Outcome Measure Comprising the Stool Frequency and Abdominal Pain Items From the Crohn’s Disease Activity Index: Psychometric Evaluation in Adults With Crohn’s Disease, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
