Monday Poster Session
Category: IBD
P2674 - Primary Prophylaxis Reduces Risk of Pouchitis Among Patients with IPAA and Primary Sclerosing Cholangitis
Monday, October 28, 2024
10:30 AM - 4:00 PM ET
Location: Exhibit Hall E
Has Audio

John P. Haydek, MD, MS
University of North Carolina at Chapel Hill
Chapel Hill, NC
Presenting Author(s)
John P. Haydek, MD, MS1, Priscila Santiago, MD2, Joseph Carter Powers, BA3, Latchmin Ruplall, 4, Hoang Anh Richardson, MD5, Erin Causey, MD5, Mohamed Hawary, MD6, Jana G. Hashash, MD, MSc7, Taha Qazi, MD8, Shannon Chang, MD9, Sara N. Horst, MD10, Frank I.. Scott, MD, MSc(Epi)11, Rami Tfayli, MD7, Millie D. Long, MD, MPH12, Laura E. Raffals, MD13, Edward Barnes, MD, MPH, FACG14
1University of North Carolina at Chapel Hill, Chapel Hill, NC; 2University of Utah, Rochester, MN; 3Cleveland Clinic Lerner College of Medicine, Cleveland, OH; 4NYU Langone Health, New York, NY; 5Vanderbilt University Medical Center, Nashville, TN; 6New York University, Smithtown, NY; 7Mayo Clinic, Jacksonville, FL; 8Cleveland Clinic Foundation, Beachwood, OH; 9New York University Langone Health, New York, NY; 10Vanderbilt Inflammatory Bowel Disease Clinic, Nashville, TN; 11University of Colorado Anschutz Medical Campus, Aurora, CO; 12Center for Gastrointestinal Biology and Disease, University of North Carolina, Chapel Hill, NC; 13Mayo Clinic, Rochester, MN; 14University of North Carolina at Chapel Hill School of Medicine, Chapel Hill, NC
Introduction: Although restorative proctocolectomy with ileal pouch-anal anastomosis (IPAA) is often depicted as a curative surgery for patients with ulcerative colitis (UC), many short and long-term complications can occur. Patients with a preoperative diagnosis of primary sclerosing cholangitis (PSC) are at a 4-times increased risk for developing pouchitis when compared to patients with UC undergoing IPAA. Despite this increased risk, there are no standardized approaches or guideline recommendations for the treatment of patients with PSC undergoing IPAA. We designed a retrospective study to address a knowledge gap regarding the role of primary prophylaxis against pouchitis among patients with PSC and UC undergoing IPAA.
Methods: A retrospective cohort was created from patients from 6 academic medical centers with a diagnosis of PSC and UC who underwent IPAA between January 1994 and May 2023. We collected data including demographics, medication use prior to IPAA, extraintestinal manifestations, and the use of primary prophylaxis medications after the final stage of IPAA. The primary outcome was a diagnosis of pouchitis at any point after IPAA. We also evaluated the development of chronic inflammatory conditions of the pouch (CP), defined as a diagnosis of chronic antibiotic-responsive pouchitis, chronic antibiotic-refractory pouchitis, or Crohn’s like disease of the pouch. Analysis was performed using descriptive statistics and one-sided Fisher’s exact test.
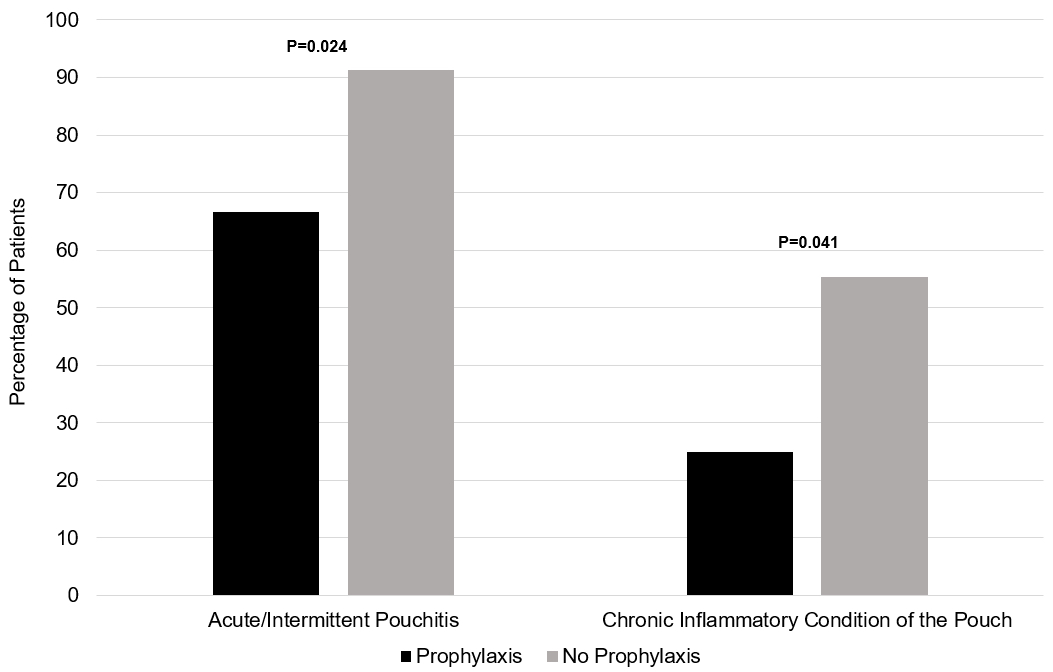
Results: Among 173 patients, 12 received primary prophylaxis (Table 1). Antibiotics were the most common prophylactic medication (5 patients), followed by probiotics (3 patients), and biologics, topical mesalamine, small molecules and immunomodulators (all 1 patient). Patients receiving any form of primary prophylaxis demonstrated significantly lower rates of acute intermittent pouchitis (66.7% vs. 91.3%, p=0.024; Figure 1) and CP (25.0% vs. 55.3%, p=0.041) when compared to patients with PSC and UC undergoing IPAA with no primary prophylaxis.
Discussion: Among patients with PSC and UC, the use of primary prophylaxis for pouchitis following IPAA creation reduced the rate of developing acute intermittent pouchitis and CP, although further subgroup analysis is limited due to very small sample sizes. Given the burden of acute intermittent and CP in this high-risk phenotype, further prospective study is needed to identify predictive factors of success with primary prophylaxis strategies and methods for implementation.
Note: The table for this abstract can be viewed in the ePoster Gallery section of the ACG 2024 ePoster Site or in The American Journal of Gastroenterology's abstract supplement issue, both of which will be available starting October 27, 2024.
Disclosures:
John P. Haydek, MD, MS1, Priscila Santiago, MD2, Joseph Carter Powers, BA3, Latchmin Ruplall, 4, Hoang Anh Richardson, MD5, Erin Causey, MD5, Mohamed Hawary, MD6, Jana G. Hashash, MD, MSc7, Taha Qazi, MD8, Shannon Chang, MD9, Sara N. Horst, MD10, Frank I.. Scott, MD, MSc(Epi)11, Rami Tfayli, MD7, Millie D. Long, MD, MPH12, Laura E. Raffals, MD13, Edward Barnes, MD, MPH, FACG14. P2674 - Primary Prophylaxis Reduces Risk of Pouchitis Among Patients with IPAA and Primary Sclerosing Cholangitis, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
1University of North Carolina at Chapel Hill, Chapel Hill, NC; 2University of Utah, Rochester, MN; 3Cleveland Clinic Lerner College of Medicine, Cleveland, OH; 4NYU Langone Health, New York, NY; 5Vanderbilt University Medical Center, Nashville, TN; 6New York University, Smithtown, NY; 7Mayo Clinic, Jacksonville, FL; 8Cleveland Clinic Foundation, Beachwood, OH; 9New York University Langone Health, New York, NY; 10Vanderbilt Inflammatory Bowel Disease Clinic, Nashville, TN; 11University of Colorado Anschutz Medical Campus, Aurora, CO; 12Center for Gastrointestinal Biology and Disease, University of North Carolina, Chapel Hill, NC; 13Mayo Clinic, Rochester, MN; 14University of North Carolina at Chapel Hill School of Medicine, Chapel Hill, NC
Introduction: Although restorative proctocolectomy with ileal pouch-anal anastomosis (IPAA) is often depicted as a curative surgery for patients with ulcerative colitis (UC), many short and long-term complications can occur. Patients with a preoperative diagnosis of primary sclerosing cholangitis (PSC) are at a 4-times increased risk for developing pouchitis when compared to patients with UC undergoing IPAA. Despite this increased risk, there are no standardized approaches or guideline recommendations for the treatment of patients with PSC undergoing IPAA. We designed a retrospective study to address a knowledge gap regarding the role of primary prophylaxis against pouchitis among patients with PSC and UC undergoing IPAA.
Methods: A retrospective cohort was created from patients from 6 academic medical centers with a diagnosis of PSC and UC who underwent IPAA between January 1994 and May 2023. We collected data including demographics, medication use prior to IPAA, extraintestinal manifestations, and the use of primary prophylaxis medications after the final stage of IPAA. The primary outcome was a diagnosis of pouchitis at any point after IPAA. We also evaluated the development of chronic inflammatory conditions of the pouch (CP), defined as a diagnosis of chronic antibiotic-responsive pouchitis, chronic antibiotic-refractory pouchitis, or Crohn’s like disease of the pouch. Analysis was performed using descriptive statistics and one-sided Fisher’s exact test.
Results: Among 173 patients, 12 received primary prophylaxis (Table 1). Antibiotics were the most common prophylactic medication (5 patients), followed by probiotics (3 patients), and biologics, topical mesalamine, small molecules and immunomodulators (all 1 patient). Patients receiving any form of primary prophylaxis demonstrated significantly lower rates of acute intermittent pouchitis (66.7% vs. 91.3%, p=0.024; Figure 1) and CP (25.0% vs. 55.3%, p=0.041) when compared to patients with PSC and UC undergoing IPAA with no primary prophylaxis.
Discussion: Among patients with PSC and UC, the use of primary prophylaxis for pouchitis following IPAA creation reduced the rate of developing acute intermittent pouchitis and CP, although further subgroup analysis is limited due to very small sample sizes. Given the burden of acute intermittent and CP in this high-risk phenotype, further prospective study is needed to identify predictive factors of success with primary prophylaxis strategies and methods for implementation.

Figure: Figure 1: Bar chart of rates of development of acute/intermittent pouchitis and chronic inflammatory conditions of the pouch, by prophylaxis status.
Note: The table for this abstract can be viewed in the ePoster Gallery section of the ACG 2024 ePoster Site or in The American Journal of Gastroenterology's abstract supplement issue, both of which will be available starting October 27, 2024.
Disclosures:
John Haydek indicated no relevant financial relationships.
Priscila Santiago indicated no relevant financial relationships.
Joseph Carter Powers: The Crohn's and Colitis Foundation Grant Funding through the Student Research Fellowship Award – Grant/Research Support.
Latchmin Ruplall indicated no relevant financial relationships.
Hoang Anh Richardson indicated no relevant financial relationships.
Erin Causey indicated no relevant financial relationships.
Mohamed Hawary indicated no relevant financial relationships.
Jana Hashash: Bristol Myers Squibb – Consultant.
Taha Qazi: Abbvie Biosciences – Advisor or Review Panel Member, Consultant, Grant/Research Support, Speakers Bureau. Celgene/BMS – Advisor or Review Panel Member, Speakers Bureau. Janssen – Speakers Bureau. Pfizer – Advisor or Review Panel Member, Advisory Committee/Board Member. Prometheus Biosciences – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant.
Shannon Chang: AbbVie – Consultant. Bristol Myers Squibb – Consultant. Janssen – Consultant. Pfizer – Consultant.
Sara Horst: AbbVie – Consultant. Bristol Myers Squibb – Consultant. Janssen – Consultant. Pfizer – Consultant. Takeda – Consultant.
Frank Scott: Crohn's and Colitis Foundation – Grant/Research Support. Takeda Pharmaceuticals – Grant/Research Support.
Rami Tfayli indicated no relevant financial relationships.
Millie Long: AbbVie – Consultant. Bristol-Myers Squibb – Consultant. Eli Lilly – Consultant, Grant/Research Support. Intercept – Consultant. Janssen – Consultant. Pfizer Inc – Consultant, Grant/Research Support. Prometheus – Consultant. Roche – Consultant. Roivant – Consultant. Takeda – Consultant, Grant/Research Support. Target RWE – Consultant.
Laura E. Raffals: Bristol Myers Squibb – Advisory Committee/Board Member, Consultant. Fresenius Kabi USA – Advisory Committee/Board Member, Consultant. Geneoscopy – Advisory Committee/Board Member, Consultant. Janssen – Advisory Committee/Board Member, Consultant. Roivant Sciences – Advisory Committee/Board Member, Consultant. Takeda – Advisory Committee/Board Member, Consultant.
Edward Barnes: AbbVie, Inc. – Consultant. Boomerang – Consultant. Bristol-Meyers Squibb – Consultant. Direct Biologics – Consultant. Eli Lilly and Company – Advisor or Review Panel Member. Pfizer – Consultant. Target RWE – Consultant.
John P. Haydek, MD, MS1, Priscila Santiago, MD2, Joseph Carter Powers, BA3, Latchmin Ruplall, 4, Hoang Anh Richardson, MD5, Erin Causey, MD5, Mohamed Hawary, MD6, Jana G. Hashash, MD, MSc7, Taha Qazi, MD8, Shannon Chang, MD9, Sara N. Horst, MD10, Frank I.. Scott, MD, MSc(Epi)11, Rami Tfayli, MD7, Millie D. Long, MD, MPH12, Laura E. Raffals, MD13, Edward Barnes, MD, MPH, FACG14. P2674 - Primary Prophylaxis Reduces Risk of Pouchitis Among Patients with IPAA and Primary Sclerosing Cholangitis, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
