Monday Poster Session
Category: IBD
P2680 - Reactive Combination Therapy by Addition of an Immunomodulator to Infliximab Monotherapy Restores Pharmacologic and Clinical Response in Inflammatory Bowel Disease: A Case Series, Systematic Review, and Meta-Analysis
Monday, October 28, 2024
10:30 AM - 4:00 PM ET
Location: Exhibit Hall E
Has Audio
Jeffrey Lowell, MD, PhD
North Shore University Hospital/Zucker School of Medicine at Hofstra University
Manhasset, NY
Presenting Author(s)
Jeffrey Lowell, MD, PhD1, Garvita Sharma, BA2, Vincent Chua, BS3, Shomron Ben-Horin, MD4, Arun Swaminath, MD5, Keith Sultan, MD, FACG3
1North Shore University Hospital/Zucker School of Medicine at Hofstra University, Manhasset, NY; 2Arkansas College of Osteopathic Medicine, Fort Smith, AR; 3Northwell Health, Manhasset, NY; 4Sheba Medical Center, Ramat Gan, Tel Aviv, Israel; 5Lenox Hill Hospital/Northwell Health, New York, NY
Introduction: Loss of response to infliximab (IFX) for inflammatory bowel disease (IBD) may occur by anti-drug antibody (ADA) development that decreases IFX levels, and can be prevented with concomitant initiation of immunomodulators (IMMs) such as methotrexate or thiopurine. However, there is limited data evaluating the impact of reactive combination therapy (rCT) in which an IMM is added to IFX monotherapy to recapture response and IFX levels following ADA development. We conducted a retrospective cohort study and systematic review with meta-analysis to examine the efficacy of rCT to recapture immunologic and clinical remission in IBD patients with rising ADAs.
Methods: IBD patients beginning IFX monotherapy between 1/1/2000-6/30/2023 who subsequently began an IMM (azathioprine, 6-mercaptopurine, or methotrexate) were identified. Also, a systematic review and meta-analysis identified studies of patients given IMM during IFX monotherapy to recapture clinical response, restore IFX trough levels, and decrease ADAs. Pooled effect size was estimated from extracted data in a random-effects model with I-squared heterogeneity assessment. ADA and trough levels were compared between those closest in date just before IMM initiation to those furthest from IMM initiation before any changes in IFX dosing or discontinuation of IMM.
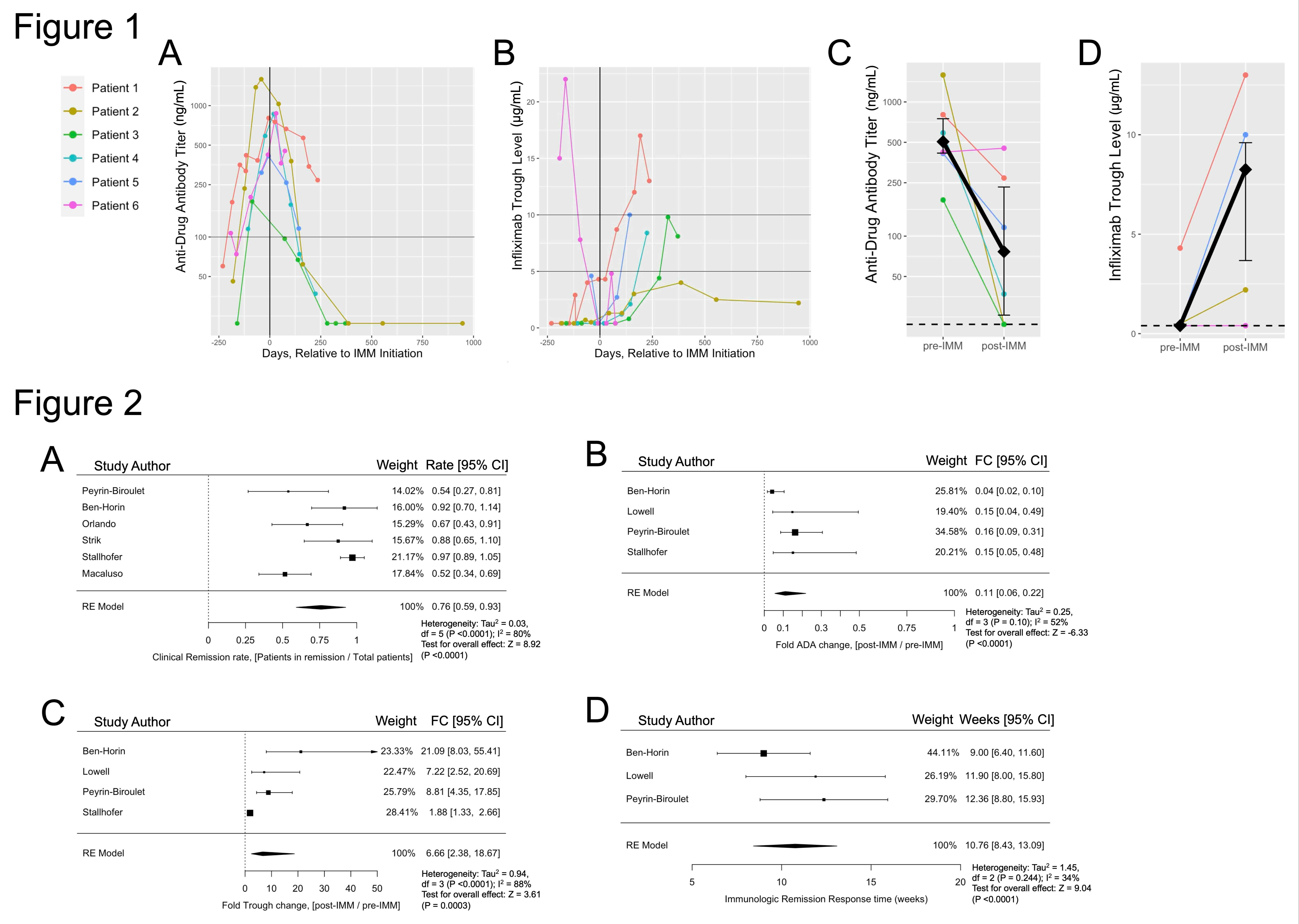
Results: We identified 6 IBD patients treated with rCT to reverse rising ADA titers and low IFX trough levels. Median ADA titer decreased from 506 ng/mL (interquartile range (IQR) [416-750]) pre-IMM to 76.5 ng/mL (IQR [25.8-232]) post-IMM, an 85% decrease (p=0.031). Median IFX serum trough increased from 0.4 µg/mL (IQR [0.4-0.48]) pre-IMM to 8.25 µg/mL (IQR [3.7-9.6]) post-IMM, a 20.6-fold increase (p=0.038) (Figure 1). By systematic review we identified 7 studies with 89 total patients treated with rCT after clinical and immunologic LOR to IFX monotherapy. Meta-analysis pooled effect size showed an 87% reduction in ADA titers (95% confidence interval [CI] 72-94%), 6.7-fold increased IFX trough (95%CI 2.4-18.7), clinical remission rescue rate of 76% (95%CI 59-93%), and 10.8-week time to immunologic response (95%CI=8.43-15.93) (Figure 2).
Discussion: These results suggest that addition of an IMM to IFX monotherapy in the context of clinical or immunologic LOR is a valid rescue strategy given its ability to reduce ADA titers, restore therapeutic IFX levels, and recapture clinical remission. Further study is necessary to compare different IMM efficacies using this treatment strategy.
Disclosures:
Jeffrey Lowell, MD, PhD1, Garvita Sharma, BA2, Vincent Chua, BS3, Shomron Ben-Horin, MD4, Arun Swaminath, MD5, Keith Sultan, MD, FACG3. P2680 - Reactive Combination Therapy by Addition of an Immunomodulator to Infliximab Monotherapy Restores Pharmacologic and Clinical Response in Inflammatory Bowel Disease: A Case Series, Systematic Review, and Meta-Analysis, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
1North Shore University Hospital/Zucker School of Medicine at Hofstra University, Manhasset, NY; 2Arkansas College of Osteopathic Medicine, Fort Smith, AR; 3Northwell Health, Manhasset, NY; 4Sheba Medical Center, Ramat Gan, Tel Aviv, Israel; 5Lenox Hill Hospital/Northwell Health, New York, NY
Introduction: Loss of response to infliximab (IFX) for inflammatory bowel disease (IBD) may occur by anti-drug antibody (ADA) development that decreases IFX levels, and can be prevented with concomitant initiation of immunomodulators (IMMs) such as methotrexate or thiopurine. However, there is limited data evaluating the impact of reactive combination therapy (rCT) in which an IMM is added to IFX monotherapy to recapture response and IFX levels following ADA development. We conducted a retrospective cohort study and systematic review with meta-analysis to examine the efficacy of rCT to recapture immunologic and clinical remission in IBD patients with rising ADAs.
Methods: IBD patients beginning IFX monotherapy between 1/1/2000-6/30/2023 who subsequently began an IMM (azathioprine, 6-mercaptopurine, or methotrexate) were identified. Also, a systematic review and meta-analysis identified studies of patients given IMM during IFX monotherapy to recapture clinical response, restore IFX trough levels, and decrease ADAs. Pooled effect size was estimated from extracted data in a random-effects model with I-squared heterogeneity assessment. ADA and trough levels were compared between those closest in date just before IMM initiation to those furthest from IMM initiation before any changes in IFX dosing or discontinuation of IMM.
Results: We identified 6 IBD patients treated with rCT to reverse rising ADA titers and low IFX trough levels. Median ADA titer decreased from 506 ng/mL (interquartile range (IQR) [416-750]) pre-IMM to 76.5 ng/mL (IQR [25.8-232]) post-IMM, an 85% decrease (p=0.031). Median IFX serum trough increased from 0.4 µg/mL (IQR [0.4-0.48]) pre-IMM to 8.25 µg/mL (IQR [3.7-9.6]) post-IMM, a 20.6-fold increase (p=0.038) (Figure 1). By systematic review we identified 7 studies with 89 total patients treated with rCT after clinical and immunologic LOR to IFX monotherapy. Meta-analysis pooled effect size showed an 87% reduction in ADA titers (95% confidence interval [CI] 72-94%), 6.7-fold increased IFX trough (95%CI 2.4-18.7), clinical remission rescue rate of 76% (95%CI 59-93%), and 10.8-week time to immunologic response (95%CI=8.43-15.93) (Figure 2).
Discussion: These results suggest that addition of an IMM to IFX monotherapy in the context of clinical or immunologic LOR is a valid rescue strategy given its ability to reduce ADA titers, restore therapeutic IFX levels, and recapture clinical remission. Further study is necessary to compare different IMM efficacies using this treatment strategy.

Figure: FIGURE 1: Change in anti-drug antibody (ADA) titer infliximab (IFX) trough level relative to time of immunomodulator (IMM) addition to IFX monotherapy. Individual patient values shown for ADA titers in (A) and IFX trough levels in (B), last values obtained prior to IMM discontinuation or change in IFX dosage or frequency. The black vertical line indicates time of immunomodulator (IMM) initiation (day 0), the x-axis depicts the relative number of days from that point, and the horizontal lines identify reference points for ADA titer of 100 ng/µL or IFX trough levels of 5 and 10 µg/mL for visualization of proposed cutoffs representing elevated ADA titers and therapeutic IFX range, respectively. Change in ADA titer (C) or IFX trough (D) representing the value of ADA titer or IFX trough obtained most immediately before initiation of IMM, shown as “pre-IMM,” and the value obtained furthest in time relative to IMM initiation but before the IMM was discontinued or the IFX dose or frequency was changed, shown as “post-IMM.” Median value is reflected by black diamond, with 95% confidence interval indicated by black vertical bars. The horizontal dashed lines in (C) and (D) figures represents the assay minimum limit of detection. Mann-Whitney U-test (n=6; fold-change ADA titer 85% decrease, p=0.031; fold-change IFX trough 20.6 increase, p=0.038). ___________________ FIGURE 2: Meta-analysis results for pooled effect size estimation using random-effects models. (A) Clinical remission rate of patients after reactive initiation of immunomodulator (IMM) to infliximab (IFX) monotherapy, 76% (0.59-0.93 95% confidence interval [CI]). (B) Relative fold change of anti-drug antibody (ADA) titers after reactive IMM addition to IFX monotherapy, 0.11 (0.06-0.22 95% CI). (C) Relative fold change of IFX trough level after reactive IMM addition to IFX monotherapy, 6.66 (2.38-18.67 95% CI). (D) Time to immunologic response measured in weeks, defined by first initial simultaneous decrease in ADA titer and IFX trough level after reactive IMM addition to IFX monotherapy, 10.76 weeks (8.43-13.09 95% CI). FC = fold change, CI = confidence interval, RE = random-effects, df = degrees of freedom.
Disclosures:
Jeffrey Lowell indicated no relevant financial relationships.
Garvita Sharma indicated no relevant financial relationships.
Vincent Chua indicated no relevant financial relationships.
Shomron Ben-Horin: AbbVie – Advisory Committee/Board Member, Consultant, Stock Options. Alma Therapeutics – Stock Options. Bristol Myers Squibb – Advisory Committee/Board Member, Consultant. Celltrion – Advisory Committee/Board Member, Consultant, Stock Options. Eli Lilly – Advisory Committee/Board Member, Consultant. Evinature – Stock Options. Ferring – Advisory Committee/Board Member, Consultant. Galmed – Advisory Committee/Board Member, Consultant, Stock Options. Gilead – Advisory Committee/Board Member, Consultant. GlaxoSmithKline – Advisory Committee/Board Member, Consultant. Janssen – Advisory Committee/Board Member, Consultant, Stock Options. Medial EarlySign – Advisory Committee/Board Member, Consultant. Medtronic – Grant/Research Support. NeoPharm – Advisory Committee/Board Member, Consultant. Novartis – Advisory Committee/Board Member, Consultant. Pfizer – Advisory Committee/Board Member, Consultant, Grant/Research Support. Predicta Med – Advisory Committee/Board Member, Consultant, Stock Options. Roche – Advisory Committee/Board Member, Consultant. Takeda – Advisory Committee/Board Member, Consultant, Grant/Research Support.
Arun Swaminath: abbvie – Grant/Research Support. janssen – Grant/Research Support.
Keith Sultan indicated no relevant financial relationships.
Jeffrey Lowell, MD, PhD1, Garvita Sharma, BA2, Vincent Chua, BS3, Shomron Ben-Horin, MD4, Arun Swaminath, MD5, Keith Sultan, MD, FACG3. P2680 - Reactive Combination Therapy by Addition of an Immunomodulator to Infliximab Monotherapy Restores Pharmacologic and Clinical Response in Inflammatory Bowel Disease: A Case Series, Systematic Review, and Meta-Analysis, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.

