Monday Poster Session
Category: IBD
P2687 - Examining the One-Year Adverse Effects of Immune Checkpoint Inhibitors Among IBD Patients With Malignancies
Monday, October 28, 2024
10:30 AM - 4:00 PM ET
Location: Exhibit Hall E
Has Audio
- SG
Sanya Goswami, MD
SUNY Downstate Health Sciences University
Brooklyn, NY
Presenting Author(s)
Sanya Goswami, MD1, Rahul Raiker, MD2, Aruj Choudhry, MD3, Farah Monzur, MD, FACG4
1SUNY Downstate Health Sciences University, Brooklyn, NY; 2George Washington University School of Medicine and Health Sciences, Washington, DC; 3Stony Brook University Hospital, Stony Brook, NY; 4Renaissance School of Medicine at Stony Brook University, Stony Brook, NY
Introduction: During the last two decades, immune checkpoint inhibitors (ICI) have markedly changed the approach to cancer therapy due to their broad bioactivity. The widespread use of ICI therapy has led to the increasing recognition of immune-related adverse events (irAEs) that range from almost every organ system. This study aims to examine the 1-year risk of ICI toxicities among IBD and non-IBD patients with malignancies. To date, ICI induced toxicities among IBD patients have not been previously described.
Methods: A retrospective cohort study was conducted using TriNetX, a multicenter database of ~100 million patients across 84 healthcare organizations. Adults from 2012-2021 were identified based on those who received ICI-therapy. Patients were then stratified by history of those with and without IBD prior to ICI initiation. ICI toxicity categories were determined a priori and included cardiac, nephrological, gastrointestinal, endocrine, respiratory, ocular, hematological, hepatological, musculoskeletal, and neurological toxicities. Patients experiencing any outcome prior to therapy were excluded from analysis. A 1:1 propensity score matching was conducted to control for comorbidities and demographics. Adjusted hazard ratios (aHR) with 95% CI were calculated at 1-year follow-up after therapy initiation for ICI toxicities between the cohorts. aHR was estimated using the Cox proportional hazard model.
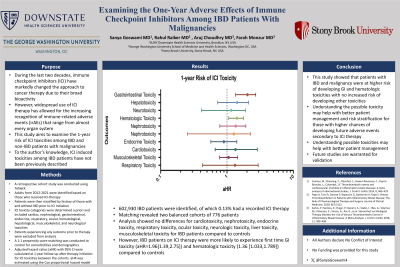
Results: 602,930 IBD patients were identified, of which 0.13% had a recorded ICI therapy. Matching revealed two balanced cohorts of 776 patients each. Analysis showed no differences for cardiotoxicity, nephrotoxicity, endocrine toxicity, respiratory toxicity, ocular toxicity, neurologic toxicity, liver toxicity, musculoskeletal toxicity for IBD patients compared to controls. However, IBD patients on ICI therapy were more likely to experience first time GI toxicity (aHR=1.96[1.39,2.75]) and hematologic toxicity (1.36 [1.033,1.789]) compared to controls.
Discussion: This study showed that patients with IBD and malignancy were at higher risk of developing GI and hematologic toxicities with no increased risk of developing other toxicities. Understanding possible toxicities may help with better patient management and risk stratification for those with higher chances of developing future adverse events secondary to ICI therapy. Future studies are warranted for validation.
Disclosures:
Sanya Goswami, MD1, Rahul Raiker, MD2, Aruj Choudhry, MD3, Farah Monzur, MD, FACG4. P2687 - Examining the One-Year Adverse Effects of Immune Checkpoint Inhibitors Among IBD Patients With Malignancies, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
1SUNY Downstate Health Sciences University, Brooklyn, NY; 2George Washington University School of Medicine and Health Sciences, Washington, DC; 3Stony Brook University Hospital, Stony Brook, NY; 4Renaissance School of Medicine at Stony Brook University, Stony Brook, NY
Introduction: During the last two decades, immune checkpoint inhibitors (ICI) have markedly changed the approach to cancer therapy due to their broad bioactivity. The widespread use of ICI therapy has led to the increasing recognition of immune-related adverse events (irAEs) that range from almost every organ system. This study aims to examine the 1-year risk of ICI toxicities among IBD and non-IBD patients with malignancies. To date, ICI induced toxicities among IBD patients have not been previously described.
Methods: A retrospective cohort study was conducted using TriNetX, a multicenter database of ~100 million patients across 84 healthcare organizations. Adults from 2012-2021 were identified based on those who received ICI-therapy. Patients were then stratified by history of those with and without IBD prior to ICI initiation. ICI toxicity categories were determined a priori and included cardiac, nephrological, gastrointestinal, endocrine, respiratory, ocular, hematological, hepatological, musculoskeletal, and neurological toxicities. Patients experiencing any outcome prior to therapy were excluded from analysis. A 1:1 propensity score matching was conducted to control for comorbidities and demographics. Adjusted hazard ratios (aHR) with 95% CI were calculated at 1-year follow-up after therapy initiation for ICI toxicities between the cohorts. aHR was estimated using the Cox proportional hazard model.
Results: 602,930 IBD patients were identified, of which 0.13% had a recorded ICI therapy. Matching revealed two balanced cohorts of 776 patients each. Analysis showed no differences for cardiotoxicity, nephrotoxicity, endocrine toxicity, respiratory toxicity, ocular toxicity, neurologic toxicity, liver toxicity, musculoskeletal toxicity for IBD patients compared to controls. However, IBD patients on ICI therapy were more likely to experience first time GI toxicity (aHR=1.96[1.39,2.75]) and hematologic toxicity (1.36 [1.033,1.789]) compared to controls.
Discussion: This study showed that patients with IBD and malignancy were at higher risk of developing GI and hematologic toxicities with no increased risk of developing other toxicities. Understanding possible toxicities may help with better patient management and risk stratification for those with higher chances of developing future adverse events secondary to ICI therapy. Future studies are warranted for validation.
Disclosures:
Sanya Goswami indicated no relevant financial relationships.
Rahul Raiker indicated no relevant financial relationships.
Aruj Choudhry indicated no relevant financial relationships.
Farah Monzur: Gather-Ed – Consultant, Speakers Bureau. Medtronic (Covidien) – Consultant. Prometheus – Consultant.
Sanya Goswami, MD1, Rahul Raiker, MD2, Aruj Choudhry, MD3, Farah Monzur, MD, FACG4. P2687 - Examining the One-Year Adverse Effects of Immune Checkpoint Inhibitors Among IBD Patients With Malignancies, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
