Monday Poster Session
Category: Liver
P2893 - Exploring Risks of Autoimmune Hepatitides and Glucocorticoid Use While on Immune Checkpoint Inhibitors: Comparative Analysis
Monday, October 28, 2024
10:30 AM - 4:00 PM ET
Location: Exhibit Hall E
Has Audio
- IT
Irakli Tskhakaia, MD
Jefferson Einstein Hospital
Philadelphia, PA
Presenting Author(s)
Irakli Tskhakaia, MD, Nino Gudushauri, MD, Irakli Lemonjava, MD, Zurab Azmaiparashvili, MD, MSc
Jefferson Einstein Hospital, Philadelphia, PA
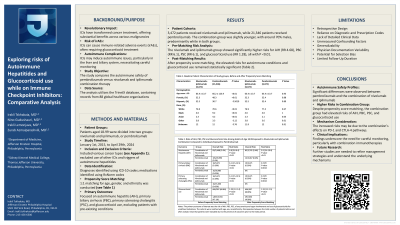
Introduction: Immune checkpoint inhibitors (ICIs) yield substantial benefits in malignancies but may cause immune-related adverse events. Autoimmune complications in the liver and biliary system have also been reported. Understanding these risks is vital for optimal patient care. In this retrospective analysis, we compare outcomes between patients treated with pembrolizumab and those receiving the combination of nivolumab and ipilimumab. Analyzing electronic health records through TrinetX database, our study aims to provide valuable insights into the autoimmune safety profiles of these immunotherapies.
Methods: Patients aged 18-99 were split into nivolumab/ipilimumab or pembrolizumab groups from Jan 1, 2015, to Apr 29, 2024. Inclusion covered various cancers, with exclusion for alternative ICIs or autoimmune hepatitis triggers. ICD-10 codes indicated diagnoses and medications were identified using RxNorm codes. Propensity score matching was conducted for age, gender, and ethnicity. The index event was defined as the onset of oncological diagnosis and ICIs appearing in records. Outcomes comprised the diagnosis of autoimmune hepatitis (AIH), primary biliary cirrhosis or cholangitis (PBC), primary sclerosing cholangitis (PSC), and the risk of glucocorticoid use, with patients with pre-existing conditions excluded from analysis.
Results: The nivolumab/ipilimumab cohort comprised 3472 individuals, while the pembrolizumab group had 21,346. Patients in the combination group were younger, whereas the pembrolizumab group exhibited a more uniform gender distribution. Both cohorts were predominantly composed of white patients. Before propensity score matching, the nivolumab and ipilimumab combination group exhibited statistically significant higher risks of AIH (RR 4.68), PBC (RR 6.1), PSC (RR 6.1) and Glucocorticoid use (RR 1.28). Post-matching, the risks remained elevated and demonstrated statistical significance (Table 1).
Discussion: Our analysis revealed higher risks of AIH, PBC, PSC, and glucocorticoid treatment needs in nivolumab and ipilimumab group compared to the pembrolizumab group. This may be attributed to the alteration of both PD-1 and CTLA-4 pathways. Results highlight the need for vigilant monitoring in immunotherapy. However, limitations such as reliance on physician documentation and diagnostic codes, alongside the retrospective nature of our analysis, should be acknowledged. Further research is essential for refining patient management and understanding underlying mechanisms.
Note: The table for this abstract can be viewed in the ePoster Gallery section of the ACG 2024 ePoster Site or in The American Journal of Gastroenterology's abstract supplement issue, both of which will be available starting October 27, 2024.
Disclosures:
Irakli Tskhakaia, MD, Nino Gudushauri, MD, Irakli Lemonjava, MD, Zurab Azmaiparashvili, MD, MSc. P2893 - Exploring Risks of Autoimmune Hepatitides and Glucocorticoid Use While on Immune Checkpoint Inhibitors: Comparative Analysis, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
Jefferson Einstein Hospital, Philadelphia, PA
Introduction: Immune checkpoint inhibitors (ICIs) yield substantial benefits in malignancies but may cause immune-related adverse events. Autoimmune complications in the liver and biliary system have also been reported. Understanding these risks is vital for optimal patient care. In this retrospective analysis, we compare outcomes between patients treated with pembrolizumab and those receiving the combination of nivolumab and ipilimumab. Analyzing electronic health records through TrinetX database, our study aims to provide valuable insights into the autoimmune safety profiles of these immunotherapies.
Methods: Patients aged 18-99 were split into nivolumab/ipilimumab or pembrolizumab groups from Jan 1, 2015, to Apr 29, 2024. Inclusion covered various cancers, with exclusion for alternative ICIs or autoimmune hepatitis triggers. ICD-10 codes indicated diagnoses and medications were identified using RxNorm codes. Propensity score matching was conducted for age, gender, and ethnicity. The index event was defined as the onset of oncological diagnosis and ICIs appearing in records. Outcomes comprised the diagnosis of autoimmune hepatitis (AIH), primary biliary cirrhosis or cholangitis (PBC), primary sclerosing cholangitis (PSC), and the risk of glucocorticoid use, with patients with pre-existing conditions excluded from analysis.
Results: The nivolumab/ipilimumab cohort comprised 3472 individuals, while the pembrolizumab group had 21,346. Patients in the combination group were younger, whereas the pembrolizumab group exhibited a more uniform gender distribution. Both cohorts were predominantly composed of white patients. Before propensity score matching, the nivolumab and ipilimumab combination group exhibited statistically significant higher risks of AIH (RR 4.68), PBC (RR 6.1), PSC (RR 6.1) and Glucocorticoid use (RR 1.28). Post-matching, the risks remained elevated and demonstrated statistical significance (Table 1).
Discussion: Our analysis revealed higher risks of AIH, PBC, PSC, and glucocorticoid treatment needs in nivolumab and ipilimumab group compared to the pembrolizumab group. This may be attributed to the alteration of both PD-1 and CTLA-4 pathways. Results highlight the need for vigilant monitoring in immunotherapy. However, limitations such as reliance on physician documentation and diagnostic codes, alongside the retrospective nature of our analysis, should be acknowledged. Further research is essential for refining patient management and understanding underlying mechanisms.
Note: The table for this abstract can be viewed in the ePoster Gallery section of the ACG 2024 ePoster Site or in The American Journal of Gastroenterology's abstract supplement issue, both of which will be available starting October 27, 2024.
Disclosures:
Irakli Tskhakaia indicated no relevant financial relationships.
Nino Gudushauri indicated no relevant financial relationships.
Irakli Lemonjava indicated no relevant financial relationships.
Zurab Azmaiparashvili indicated no relevant financial relationships.
Irakli Tskhakaia, MD, Nino Gudushauri, MD, Irakli Lemonjava, MD, Zurab Azmaiparashvili, MD, MSc. P2893 - Exploring Risks of Autoimmune Hepatitides and Glucocorticoid Use While on Immune Checkpoint Inhibitors: Comparative Analysis, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
