Tuesday Poster Session
Category: IBD
P4307 - Corticosteroid Sparing Effects of Treatment With Guselkumab in Patients With Moderate to Severely Active Ulcerative Colitis: Phase 3 QUASAR Maintenance Study Results Through Week 44
Tuesday, October 29, 2024
10:30 AM - 4:00 PM ET
Location: Exhibit Hall E
Has Audio

Brian Bressler, MS, MD
University of British Columbia, IBD Centre of BC
Vancouver, BC, Canada
Presenting Author(s)
Brian Bressler, MS, MD1, Jessica R. Allegretti, MD, MPH, FACG2, David T. Rubin, MD, FACG3, Nicole Shipitofsky, PharmD4, Kuan-Hsiang G. Huang, MD, PhD4, Matthew Germinaro, MD4, Rebbecca Wilson, 5, Hongyan Zhang, PhD4, Alessandro Armuzzi, MD, PhD6, Sigal Fishman, MD7, Yufang Wang, MD8, Julián Panés, MD9, Gary R. Lichtenstein, MD, FACG10, Laurent Peyrin-Biroulet, MD, PhD11
1University of British Columbia, IBD Centre of BC, Vancouver, BC, Canada; 2Brigham and Women’s Hospital, Harvard Medical School, Boston, MA; 3University of Chicago Medicine Inflammatory Bowel Disease Center, Chicago, IL; 4Janssen Research and Development, Spring House, PA; 5Janssen Research and Development, LLC, Spring House, PA; 6IRCCS Humanitas Research Hospital, Milan, Lombardia, Italy; 7Sourasky Medical Center, Tel Aviv, Tel Aviv, Israel; 8West China Hospital of Sichuan University, Chengdu, Sichuan, China; 9Hospital Clínic de Barcelona, IDIBAPS, CIBERehd, Barcelona, Catalonia, Spain; 10Perelman Center for Advanced Medicine, University of Pennsylvania, Philadelphia, PA; 11INFINY Institute, FHU-CURE, INSERM NGERE, Nancy University Hospital, Vandœuvre-lès-Nancy, Lorraine, France; McGill University Health Centre, Montreal, QC, Canada, Nancy, Lorraine, France
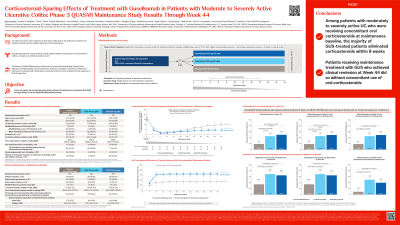
Introduction: The Phase 3 QUASAR Maintenance Study (NCT04033445) evaluated the efficacy of maintenance treatment with SC guselkumab (GUS), a dual-acting IL-23p19 subunit inhibitor that binds to CD64, in patients (pts) with ulcerative colitis (UC). Here we report the corticosteroid sparing effects of maintenance treatment with GUS compared with GUS withdrawal (placebo [PBO]) through Week (Wk) 44.
Methods: At maintenance baseline, clinical responders to 12 wks of GUS IV induction were randomized 1:1:1 to GUS 200 mg SC q4w, GUS 100 mg SC q8w, or GUS withdrawal (PBO). Pts who were receiving oral corticosteroids upon entry into the maintenance study were required to begin tapering their daily corticosteroid dose at Wk0, unless medically not feasible. Corticosteroid use and corticosteroid-free clinical remission at Wk44 were assessed.
Results: The primary analysis population included 568 pts (induction baseline: mean age, 40.7yr; mean UC duration, 7.8yr; mean modified Mayo score, 6.9 [63.9% with severe disease]; Mayo endoscopy subscore of 3, 66.4%). At maintenance baseline, 38.9% (221/568) of pts were receiving oral corticosteroids.
Among pts who were receiving oral corticosteroids (excluding budesonide and beclomethasone dipropionate) at maintenance baseline, the mean decreases from baseline in the average daily prednisone-equivalent corticosteroid dose at Wk44 were greater in the GUS 100mg and 200mg groups compared with the withdrawal group (-10.22 and -10.25 vs -7.35mg/day, respectively, both nominal p< 0.001). As early as Wk8, higher proportions of pts in both GUS treatment groups had eliminated oral corticosteroids compared with the withdrawal group (65.8% [48/73] and 64.4% [47/73] for GUS 100mg and GUS 200mg groups, respectively, vs 32.0% [24/75] for withdrawal group, both nominal p< 0.001). At Wk44, higher proportions of pts in the GUS treatment groups vs withdrawal group had eliminated oral corticosteroids (Table 1). Higher proportions of pts in the GUS treatment groups were in clinical remission at Wk44 and had eliminated oral corticosteroids for at least 8 wks prior to Wk44, compared with the withdrawal group (Table 1).
Among GUS-treated pts, 180 achieved clinical remission at Wk44 and 178 (98.9%) of them had eliminated oral corticosteroids at least 8 wks prior to Wk44.
Discussion: In patients with UC, maintenance treatment with GUS 100mg SC q8w and GUS 200mg SC q4w sustained clinically meaningful efficacy through Wk44 and allowed successful elimination of concomitant corticosteroids.
Note: The table for this abstract can be viewed in the ePoster Gallery section of the ACG 2024 ePoster Site or in The American Journal of Gastroenterology's abstract supplement issue, both of which will be available starting October 27, 2024.
Disclosures:
Brian Bressler, MS, MD1, Jessica R. Allegretti, MD, MPH, FACG2, David T. Rubin, MD, FACG3, Nicole Shipitofsky, PharmD4, Kuan-Hsiang G. Huang, MD, PhD4, Matthew Germinaro, MD4, Rebbecca Wilson, 5, Hongyan Zhang, PhD4, Alessandro Armuzzi, MD, PhD6, Sigal Fishman, MD7, Yufang Wang, MD8, Julián Panés, MD9, Gary R. Lichtenstein, MD, FACG10, Laurent Peyrin-Biroulet, MD, PhD11. P4307 - Corticosteroid Sparing Effects of Treatment With Guselkumab in Patients With Moderate to Severely Active Ulcerative Colitis: Phase 3 QUASAR Maintenance Study Results Through Week 44, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
1University of British Columbia, IBD Centre of BC, Vancouver, BC, Canada; 2Brigham and Women’s Hospital, Harvard Medical School, Boston, MA; 3University of Chicago Medicine Inflammatory Bowel Disease Center, Chicago, IL; 4Janssen Research and Development, Spring House, PA; 5Janssen Research and Development, LLC, Spring House, PA; 6IRCCS Humanitas Research Hospital, Milan, Lombardia, Italy; 7Sourasky Medical Center, Tel Aviv, Tel Aviv, Israel; 8West China Hospital of Sichuan University, Chengdu, Sichuan, China; 9Hospital Clínic de Barcelona, IDIBAPS, CIBERehd, Barcelona, Catalonia, Spain; 10Perelman Center for Advanced Medicine, University of Pennsylvania, Philadelphia, PA; 11INFINY Institute, FHU-CURE, INSERM NGERE, Nancy University Hospital, Vandœuvre-lès-Nancy, Lorraine, France; McGill University Health Centre, Montreal, QC, Canada, Nancy, Lorraine, France
Introduction: The Phase 3 QUASAR Maintenance Study (NCT04033445) evaluated the efficacy of maintenance treatment with SC guselkumab (GUS), a dual-acting IL-23p19 subunit inhibitor that binds to CD64, in patients (pts) with ulcerative colitis (UC). Here we report the corticosteroid sparing effects of maintenance treatment with GUS compared with GUS withdrawal (placebo [PBO]) through Week (Wk) 44.
Methods: At maintenance baseline, clinical responders to 12 wks of GUS IV induction were randomized 1:1:1 to GUS 200 mg SC q4w, GUS 100 mg SC q8w, or GUS withdrawal (PBO). Pts who were receiving oral corticosteroids upon entry into the maintenance study were required to begin tapering their daily corticosteroid dose at Wk0, unless medically not feasible. Corticosteroid use and corticosteroid-free clinical remission at Wk44 were assessed.
Results: The primary analysis population included 568 pts (induction baseline: mean age, 40.7yr; mean UC duration, 7.8yr; mean modified Mayo score, 6.9 [63.9% with severe disease]; Mayo endoscopy subscore of 3, 66.4%). At maintenance baseline, 38.9% (221/568) of pts were receiving oral corticosteroids.
Among pts who were receiving oral corticosteroids (excluding budesonide and beclomethasone dipropionate) at maintenance baseline, the mean decreases from baseline in the average daily prednisone-equivalent corticosteroid dose at Wk44 were greater in the GUS 100mg and 200mg groups compared with the withdrawal group (-10.22 and -10.25 vs -7.35mg/day, respectively, both nominal p< 0.001). As early as Wk8, higher proportions of pts in both GUS treatment groups had eliminated oral corticosteroids compared with the withdrawal group (65.8% [48/73] and 64.4% [47/73] for GUS 100mg and GUS 200mg groups, respectively, vs 32.0% [24/75] for withdrawal group, both nominal p< 0.001). At Wk44, higher proportions of pts in the GUS treatment groups vs withdrawal group had eliminated oral corticosteroids (Table 1). Higher proportions of pts in the GUS treatment groups were in clinical remission at Wk44 and had eliminated oral corticosteroids for at least 8 wks prior to Wk44, compared with the withdrawal group (Table 1).
Among GUS-treated pts, 180 achieved clinical remission at Wk44 and 178 (98.9%) of them had eliminated oral corticosteroids at least 8 wks prior to Wk44.
Discussion: In patients with UC, maintenance treatment with GUS 100mg SC q8w and GUS 200mg SC q4w sustained clinically meaningful efficacy through Wk44 and allowed successful elimination of concomitant corticosteroids.
Note: The table for this abstract can be viewed in the ePoster Gallery section of the ACG 2024 ePoster Site or in The American Journal of Gastroenterology's abstract supplement issue, both of which will be available starting October 27, 2024.
Disclosures:
Brian Bressler: AbbVie – Advisory Committee/Board Member, Grant/Research Support, Speakers Bureau. Alimentiv – Advisory Committee/Board Member. Allergan – Advisory Committee/Board Member. Amgen – Advisory Committee/Board Member, Grant/Research Support. AMT – Advisory Committee/Board Member. Bausch Health – Advisory Committee/Board Member. BMS – Advisory Committee/Board Member, Grant/Research Support, Speakers Bureau. Boehringer Ingelheim – Grant/Research Support. Celgene – Advisory Committee/Board Member. Celltrion – Advisory Committee/Board Member. Eupraxia – Advisory Committee/Board Member. Fresenius Kabi – Advisory Committee/Board Member. Genentech/Roche – Advisory Committee/Board Member, Grant/Research Support. Gilead – Advisory Committee/Board Member. GSK – Grant/Research Support. Iterative Scopes – Advisory Committee/Board Member. Jamp – Advisor or Review Panel Member. Janssen – Advisory Committee/Board Member, Grant/Research Support, Speakers Bureau. Merck – Advisor or Review Panel Member, Grant/Research Support, Speakers Bureau. Microbiome Insights – Advisor or Review Panel Member. Mylan – Advisor or Review Panel Member. Novartis – Advisory Committee/Board Member, Speakers Bureau. Organon – Advisory Committee/Board Member, Speakers Bureau. Pendopharm – Advisor or Review Panel Member. Pfizer – Advisory Committee/Board Member, Speakers Bureau. Protagonist – Advisor or Review Panel Member. Qu Biologic – Grant/Research Support, Stock Options. Sandoz – Advisory Committee/Board Member, Speakers Bureau. Takeda – Advisory Committee/Board Member, Speakers Bureau. Viatris – Advisor or Review Panel Member.
Jessica R. Allegretti: Abbvie – Consultant, Speakers Bureau. Artugen – Consultant. Bristol Myers Squibb – Consultant, Speakers Bureau. Ferring – Consultant. Finch Therapeutics – Consultant. Janssen – Consultant. Merck – Grant/Research Support. Pfizer – Consultant. Seres – Consultant.
David T. Rubin: AbbVie – Consultant. Altrubio – Consultant. Apex – Consultant. Avalo Therapeutics – Consultant. Boehringer Ingelheim – Consultant. Bristol-Myers Squibb – Consultant. Buhlmann Diagnostics Corp – Consultant. Celgene – Consultant. Connect BioPharma – Consultant. Intouch Group – Consultant. Iterative Health – Consultant. Janssen – Consultant. Lilly – Consultant. Pfizer – Consultant. Samsung Neurologica – Consultant. Takeda – Consultant, Grant/Research Support.
Nicole Shipitofsky: Johnson & Johnson – Employee, Stock Options, Stock-publicly held company(excluding mutual/index funds).
Kuan-Hsiang G. Huang: Johnson & Johnson – Employee, Stock Options, Stock-publicly held company(excluding mutual/index funds).
Matthew Germinaro: Johnson & Johnson – Employee, Stock Options, Stock-publicly held company(excluding mutual/index funds).
Rebbecca Wilson: Johnson & Johnson – Employee, Stock Options, Stock-publicly held company(excluding mutual/index funds).
Hongyan Zhang: Johnson & Johnson – Employee, Stock Options, Stock-publicly held company(excluding mutual/index funds).
Alessandro Armuzzi: AbbVie – Advisory Committee/Board Member, Consultant, Speakers Bureau. AG Pharma – Speakers Bureau. Amgen – Advisory Committee/Board Member, Consultant, Speakers Bureau. Biogen – Advisory Committee/Board Member, Consultant, Grant/Research Support, Speakers Bureau. Boehringer Ingelheim – Advisory Committee/Board Member, Consultant. Bristol Myers Squibb – Advisory Committee/Board Member, Consultant, Speakers Bureau. Celltrion – Advisory Committee/Board Member, Consultant, Speakers Bureau. Eli-Lilly – Advisory Committee/Board Member, Consultant, Speakers Bureau. Ferring – Advisory Committee/Board Member, Consultant, Speakers Bureau. Galapagos – Advisory Committee/Board Member, Consultant, Speakers Bureau. Gilead – Advisory Committee/Board Member, Consultant, Speakers Bureau. Janssen – Advisory Committee/Board Member, Consultant, Speakers Bureau. Lionhealth – Advisory Committee/Board Member, Consultant. MSD – Advisory Committee/Board Member, Consultant, Grant/Research Support, Speakers Bureau. Mylan – Advisory Committee/Board Member, Consultant. Nestlé – Advisory Committee/Board Member, Consultant. Novartis – Speakers Bureau. Pfizer – Advisory Committee/Board Member, Consultant, Grant/Research Support, Speakers Bureau. Protagonist Therapeutics – Advisory Committee/Board Member, Consultant. Roche – Advisory Committee/Board Member, Consultant, Speakers Bureau. Samsung Bioepis – Advisory Committee/Board Member, Consultant, Speakers Bureau. Sandoz – Advisory Committee/Board Member, Consultant, Speakers Bureau. Takeda – Advisory Committee/Board Member, Consultant, Grant/Research Support, Speakers Bureau. Teva Pharmaceuticals – Speakers Bureau. Tillots Pharma – Advisory Committee/Board Member, Consultant.
Sigal Fishman: Janssen – Clinical Investigator.
Yufang Wang: Janssen – Clinical Investigator.
Julián Panés: AbbVie – Consultant, Honorarium. Alimentiv – Advisory Committee/Board Member, Consultant, Honorarium. Athos – Consultant, Honorarium. Atomwise – Consultant, Honorarium. Boehringer Ingelheim – Consultant, Honorarium. Celsius – Consultant, Honorarium. Ferring – Consultant, Honorarium. Galapagos – Consultant, Honorarium. Genentech/Roche – Consultant, Honorarium. GlaxoSmithKline – Consultant, Honorarium. Janssen – Consultant, Honorarium. Mirum – Advisory Committee/Board Member, Consultant, honorarium. Nimbus – Consultant, Honorarium. Pfizer – Consultant, Honorarium. Progenity – Consultant, Honorarium. Prometheus – Consultant, Honorarium. Protagonist – Consultant, Honorarium. Revolo – Consultant, Honorarium. Sanofi – Advisory Committee/Board Member, Consultant, Honorarium. Sorriso – Advisory Committee/Board Member, Consultant, Honorarium. Surrozen – Advisory Committee/Board Member, Consultant, Honorarium. Takeda – Consultant, Honorarium. Wasserman – Consultant, Honorarium.
Gary R. Lichtenstein: AbbVie – Consultant. American College of Gastroenterology – honoraria. American Gastroenterological Association – CME. American Regent – Consultant, Honorarium. Celgene – Consultant, Grant/Research Support. Cellceutix – Consultant. Chemed – CME. Eli Lilly – Advisory Committee/Board Member, Consultant. Endo – Consultant. Ferring – Consultant. Gastroenterology & Hepatology – Gastro-Hep Communication, Editor- Honorarium. Gilead – Consultant. IMEDEX – CME. Ironwood – CME. Janssen – Consultant, Grant/Research Support. MedEd Consultants – Consultant. Merck – Consultant, Honorarium. Morphic Therapeutics – Consultant. Pfizer – Consultant, Grant/Research Support. Professional Communications Inc. – Royalties. Prometheus Laboratories – Consultant. Romark – Consultant, honoraria. Salix/Valeant – Consultant. Sandoz – Consultant. Shire – Consultant. SLACK Inc. – Royalties. Springer Science and Business Media – Honorarium. Takeda – Consultant, Grant/Research Support. UCB – Consultant, Grant/Research Support. University of Kentucky – CME. UpToDate – honoraria. Vindico – CME. Virgo – Consultant, Stock Options.
Laurent Peyrin-Biroulet: AbbVie – Grant/Research Support, Personal fees. Allergan – Personal Fees. Alma Bio Therapeutics – Personal Fees. Amgen – Personal Fees. Applied Molecular Transport – Personal Fees. Arena – Personal Fees. Biogen – Personal Fees. Boehringer Ingelheim – Personal Fees. Bristol Myers Squibb – Personal Fees. Celgene – Personal Fees. Celltrion – Personal Fees. CTMA – Stock Options. Enterome – Personal Fees. Enthera – Personal Fees. Ferring – Personal Fees. Fresenius Kabi – Personal Fees. Genentech – Personal Fees. Gilead – Personal Fees. Hikma – Personal Fees. InDex Pharmaceuticals – Personal Fees. Janssen – Personal Fees. Lilly – Personal Fees. MSD – Grant/Research Support, Personal Fees. Mylan – Personal Fees. Nestlé – Personal Fees. Norgine – Personal Fees. Oppilan Pharma – Personal Fees. OSE Immunotherapeutics – Personal Fees. Pfizer Inc – Personal Fees. Pharmacosmos – Fees. Samsung Bioepis – Personal Fees. Sandoz – Personal Fees. Sterna – Personal Fees. Sublimity Therapeutics – Personal Fees. Takeda – Grant/Research Support, Personal Fees. Tillotts – Personal Fees. Vifor – Personal Fees.
Brian Bressler, MS, MD1, Jessica R. Allegretti, MD, MPH, FACG2, David T. Rubin, MD, FACG3, Nicole Shipitofsky, PharmD4, Kuan-Hsiang G. Huang, MD, PhD4, Matthew Germinaro, MD4, Rebbecca Wilson, 5, Hongyan Zhang, PhD4, Alessandro Armuzzi, MD, PhD6, Sigal Fishman, MD7, Yufang Wang, MD8, Julián Panés, MD9, Gary R. Lichtenstein, MD, FACG10, Laurent Peyrin-Biroulet, MD, PhD11. P4307 - Corticosteroid Sparing Effects of Treatment With Guselkumab in Patients With Moderate to Severely Active Ulcerative Colitis: Phase 3 QUASAR Maintenance Study Results Through Week 44, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
