Tuesday Poster Session
Category: IBD
P4354 - Long-Term Endoscopic and Histological Outcomes of Mirikizumab in Patients With Moderately to Severely Active Ulcerative Colitis With up to 3 Years of Treatment
Tuesday, October 29, 2024
10:30 AM - 4:00 PM ET
Location: Exhibit Hall E

Has Audio

Bruce E. Sands, MD, FACG
Icahn School of Medicine at Mount Sinai
New York, NY
Presenting Author(s)
Bruce E.. Sands, MD, FACG1, Taku Kobayashi, MD2, Jianmin Wu, 3, Isabella Yali Wang, 3, Baojin Zhu, 3, Isabel Redondo, MD4, Laurent Peyrin-Biroulet, MD, PhD5, Alissa Walsh, MD6, Fernando Magro, MD, PhD7
1Icahn School of Medicine at Mount Sinai, New York, NY; 2Kitasato University Kitasato Institute Hospital, Minato City, Tokyo, Japan; 3Eli Lilly and Company, Indianapolis, IN; 4Eli Lilly and Company, Lisboa, Lisboa, Portugal; 5INFINY Institute, FHU-CURE, INSERM NGERE, Nancy University Hospital, Vandœuvre-lès-Nancy, Lorraine, France; McGill University Health Centre, Montreal, QC, Canada, Nancy, Lorraine, France; 6Translational Gastroenterology Unit, Oxford University Hospital, Oxford, England, United Kingdom; 7University of Porto and Centro Hospitalar, Porto, Porto, Portugal
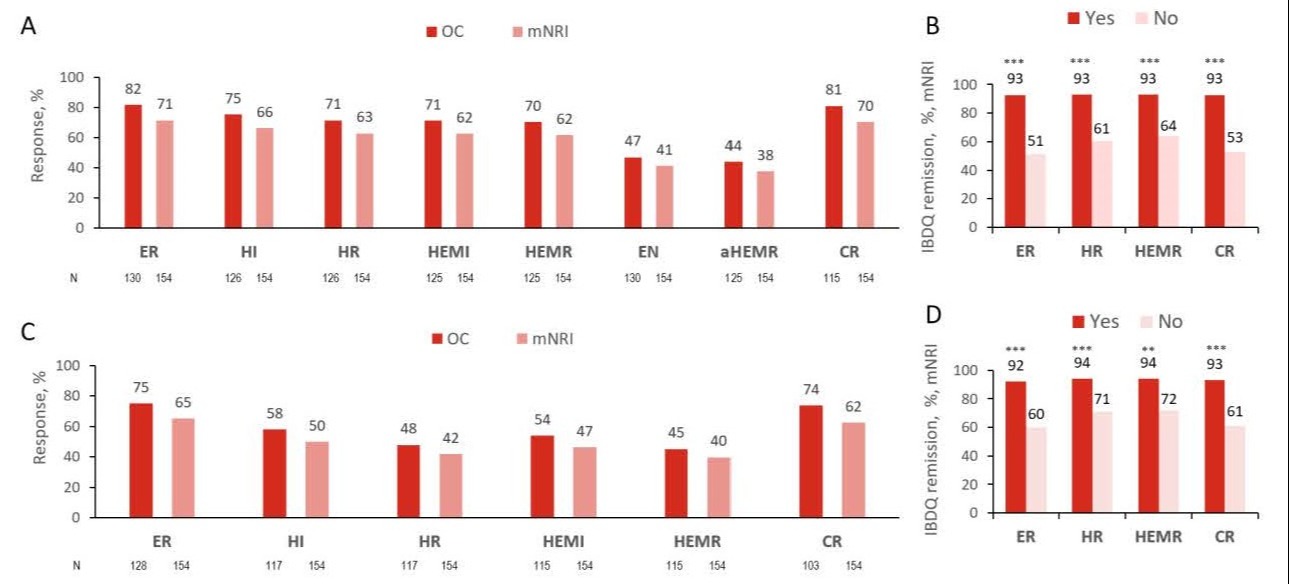
Introduction: Mirikizumab (MIRI), achieved the primary endpoint of clinical remission (CR) and other key secondary outcomes, including endoscopic and histologic improvement and remission at week (W) 12 of LUCENT-1 induction trial (NCT03518086), W52 of LUCENT-2 maintenance trial (NCT03524092), and W104 of LUCENT-3 long-term (LT) extension study (NCT03519945) in patients (pts) with moderately to severely active ulcerative colitis (UC).1-4 Here we present the LT durable and sustained histological and endoscopic outcomes through 3 years and their association with quality of life.
Methods: LUCENT-3 is an ongoing single-arm, open-label, LT extension phase 3 study evaluating the efficacy and safety of MIRI in pts who participated in LUCENT-1 and -2. For pts who achieved CR at W52 (clinical remitters), we evaluated the proportion who achieved histologic improvement or remission (HI or HR), endoscopic remission or normalization (ER or EN), histologic-endoscopic mucosal improvement or remission (HEMI or HEMR), alternate HEMR (aHEMR: Geboes score≤ 2B.0 plus Mayo ES=0) or CR at W152 and maintenance of these endpoints from W52 to W104, and W152. The relationship between clinical outcomes and Inflammatory Bowel Disease Questionnaire (IBDQ) remission (defined as IBDQ score ≥170) was assessed at W152.
Results: With 3 years of continuous MIRI treatment, the proportion of pts achieving ER, HI, HR, HEMI, HEMR, EN, aHEMR, and CR at W152 were 82%, 75%, 71%, 71%, 70%, 47%, 44%, and 81%, respectively (observed data) (Fig. A). Achieving these outcomes was associated with IBDQ remission (p< 0.001) (Fig. B). The proportion of pts who maintained ER, HI, HR, HEMI, HEMR, and CR across W52, W104, and W152 were 75%, 58%, 48%, 54%, 45%, and 74% (observed data) (Fig. C). Greater than 90% of patients who maintained these outcomes at all timepoints during 3 years with MIRI achieved IBDQ remission (Fig. D).
Discussion: MIRI maintained endoscopic and histologic outcomes through an additional 2 years of continuous treatment among clinical remitters at W52 (3 years in total). The achievement of LT durable and sustained endoscopic and histologic outcomes with MIRI was strongly associated with IBDQ remission.
References
1. D'Haens G, et al. Gastroenterology. 2022;162(Suppl_7):S214.
2. Dubinsky MC, et al. Gastroenterology. 2022;162(7, Suppl_7):S1393-S1394.
3. Magro F, et al. United European Gastroenterology J. 2023;11(Suppl_8).
4. Sands BE, et al. Inflamm Bowel Dis. 2024;izae024.
Disclosures:
Bruce E.. Sands, MD, FACG1, Taku Kobayashi, MD2, Jianmin Wu, 3, Isabella Yali Wang, 3, Baojin Zhu, 3, Isabel Redondo, MD4, Laurent Peyrin-Biroulet, MD, PhD5, Alissa Walsh, MD6, Fernando Magro, MD, PhD7. P4354 - Long-Term Endoscopic and Histological Outcomes of Mirikizumab in Patients With Moderately to Severely Active Ulcerative Colitis With up to 3 Years of Treatment, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
1Icahn School of Medicine at Mount Sinai, New York, NY; 2Kitasato University Kitasato Institute Hospital, Minato City, Tokyo, Japan; 3Eli Lilly and Company, Indianapolis, IN; 4Eli Lilly and Company, Lisboa, Lisboa, Portugal; 5INFINY Institute, FHU-CURE, INSERM NGERE, Nancy University Hospital, Vandœuvre-lès-Nancy, Lorraine, France; McGill University Health Centre, Montreal, QC, Canada, Nancy, Lorraine, France; 6Translational Gastroenterology Unit, Oxford University Hospital, Oxford, England, United Kingdom; 7University of Porto and Centro Hospitalar, Porto, Porto, Portugal
Introduction: Mirikizumab (MIRI), achieved the primary endpoint of clinical remission (CR) and other key secondary outcomes, including endoscopic and histologic improvement and remission at week (W) 12 of LUCENT-1 induction trial (NCT03518086), W52 of LUCENT-2 maintenance trial (NCT03524092), and W104 of LUCENT-3 long-term (LT) extension study (NCT03519945) in patients (pts) with moderately to severely active ulcerative colitis (UC).1-4 Here we present the LT durable and sustained histological and endoscopic outcomes through 3 years and their association with quality of life.
Methods: LUCENT-3 is an ongoing single-arm, open-label, LT extension phase 3 study evaluating the efficacy and safety of MIRI in pts who participated in LUCENT-1 and -2. For pts who achieved CR at W52 (clinical remitters), we evaluated the proportion who achieved histologic improvement or remission (HI or HR), endoscopic remission or normalization (ER or EN), histologic-endoscopic mucosal improvement or remission (HEMI or HEMR), alternate HEMR (aHEMR: Geboes score≤ 2B.0 plus Mayo ES=0) or CR at W152 and maintenance of these endpoints from W52 to W104, and W152. The relationship between clinical outcomes and Inflammatory Bowel Disease Questionnaire (IBDQ) remission (defined as IBDQ score ≥170) was assessed at W152.
Results: With 3 years of continuous MIRI treatment, the proportion of pts achieving ER, HI, HR, HEMI, HEMR, EN, aHEMR, and CR at W152 were 82%, 75%, 71%, 71%, 70%, 47%, 44%, and 81%, respectively (observed data) (Fig. A). Achieving these outcomes was associated with IBDQ remission (p< 0.001) (Fig. B). The proportion of pts who maintained ER, HI, HR, HEMI, HEMR, and CR across W52, W104, and W152 were 75%, 58%, 48%, 54%, 45%, and 74% (observed data) (Fig. C). Greater than 90% of patients who maintained these outcomes at all timepoints during 3 years with MIRI achieved IBDQ remission (Fig. D).
Discussion: MIRI maintained endoscopic and histologic outcomes through an additional 2 years of continuous treatment among clinical remitters at W52 (3 years in total). The achievement of LT durable and sustained endoscopic and histologic outcomes with MIRI was strongly associated with IBDQ remission.
References
1. D'Haens G, et al. Gastroenterology. 2022;162(Suppl_7):S214.
2. Dubinsky MC, et al. Gastroenterology. 2022;162(7, Suppl_7):S1393-S1394.
3. Magro F, et al. United European Gastroenterology J. 2023;11(Suppl_8).
4. Sands BE, et al. Inflamm Bowel Dis. 2024;izae024.

Figure: Figure. Long-term durable and sustained histological and endoscopic outcomes through 3 years and their association with IBDQ remission.
A. Proportion of patients on MIRI achieving ER, HI, HR, HEMI, HEMR, EN, aHEMR, and CR at W152 among clinical remitters at W52.
B. Association between IBDQ remission and ER, HR, HEMR and ER at W152.
C. Proportion of patients on MIRI maintaining ER, HI, HR, HEMI, HEMR and CR across W52, W104 and W152.
D. Association between IBDQ remission at W152 and maintaining ER, HR, HEMR and CR with MIRI treatment across W52, W104 and W152.
**p<0.01; ***p<0.001: Yes vs NO achieving the targeted endpoint. P-values on associations were estimated using the chi-squared test. Missing data were imputed using mNRI, in addition to the observed data. IBDQ remission was defined as IBDQ score ≥170. All time points refer to the total duration of continuous mirikizumab treatment.
aHEMR=alternate HEMR (Geboes score≤ 2B.0 + Mayo ES=0); CI=confidence interval; CR=clinical remission (MMS SF=0 or SF=1 with ≥1-point decrease from baseline; RB=0; ES=0 or 1 [excluding friability]); EN=endoscopic normalization (Mayo ES=0); ER=endoscopic remission (Mayo ES = 0 or 1 [excluding friability]); ES= endoscopic score; HEMI=histologic-endoscopic mucosal improvement (Mayo ES=0 or 1 [excluding friability] + Geboes score≤ 3.1); HEMR=histologic-endoscopic mucosal remission (Mayo ES=0 or 1 [excluding friability] + Geboes score≤ 2B.0); HI=histologic improvement (Geboes score≤ 3.1); HR=histologic remission (Geboes score≤ 2B.0); IBDQ=Inflammatory Bowel Disease Questionnaire; MMS=Mayo Modified Score; mNRI=modified nonresponder imputation; N=number of participants in the analysis population; NA=not available; OC=observed case; RB=rectal bleeding; SF=stool frequency; W=Week
A. Proportion of patients on MIRI achieving ER, HI, HR, HEMI, HEMR, EN, aHEMR, and CR at W152 among clinical remitters at W52.
B. Association between IBDQ remission and ER, HR, HEMR and ER at W152.
C. Proportion of patients on MIRI maintaining ER, HI, HR, HEMI, HEMR and CR across W52, W104 and W152.
D. Association between IBDQ remission at W152 and maintaining ER, HR, HEMR and CR with MIRI treatment across W52, W104 and W152.
**p<0.01; ***p<0.001: Yes vs NO achieving the targeted endpoint. P-values on associations were estimated using the chi-squared test. Missing data were imputed using mNRI, in addition to the observed data. IBDQ remission was defined as IBDQ score ≥170. All time points refer to the total duration of continuous mirikizumab treatment.
aHEMR=alternate HEMR (Geboes score≤ 2B.0 + Mayo ES=0); CI=confidence interval; CR=clinical remission (MMS SF=0 or SF=1 with ≥1-point decrease from baseline; RB=0; ES=0 or 1 [excluding friability]); EN=endoscopic normalization (Mayo ES=0); ER=endoscopic remission (Mayo ES = 0 or 1 [excluding friability]); ES= endoscopic score; HEMI=histologic-endoscopic mucosal improvement (Mayo ES=0 or 1 [excluding friability] + Geboes score≤ 3.1); HEMR=histologic-endoscopic mucosal remission (Mayo ES=0 or 1 [excluding friability] + Geboes score≤ 2B.0); HI=histologic improvement (Geboes score≤ 3.1); HR=histologic remission (Geboes score≤ 2B.0); IBDQ=Inflammatory Bowel Disease Questionnaire; MMS=Mayo Modified Score; mNRI=modified nonresponder imputation; N=number of participants in the analysis population; NA=not available; OC=observed case; RB=rectal bleeding; SF=stool frequency; W=Week
Disclosures:
Bruce Sands: AbbVie – Consultant. Abivax – Consultant, Speakers Bureau. Adiso Therapeutics – Consultant. Agomab – Consultant. Alimentiv – Consultant. Amgen – Consultant. AnaptysBio – Consultant. Arena Pharmaceuticals – Consultant. Artugen Therapeutics – Consultant. AstraZeneca – Consultant. Biolojic Design – Consultant. Biora Therapeutics – Consultant. Boehringer Ingelheim – Consultant. Boston Pharmaceuticals – Consultant. Bristol Myers Squibb – Consultant, Grant/Research Support, Other support, Speakers Bureau. Calibr – Consultant. Celgene – Consultant. Celltrion – Consultant. ClostraBio – Consultant. Enthera – Consultant. Envied Biosciences – Consultant. Equilium – Consultant. Evommune – Consultant. Ferring – Consultant. Fiat – Consultant. Fresenius Kabi – Consultant. Galapagos – Consultant. Genentech (Roche) – Consultant. Gilead Sciences – Consultant. Glaxo SmithKline – Consultant. Gossamer Bio – Consultant. Imhotex – Consultant. Index Pharmaceuticals – Consultant. Innovation Pharmaceuticals – Consultant. Inotrem – Consultant. Janssen – Consultant, Grant/Research Support, Other support, Speakers Bureau. Kaleido – Consultant. Kallyope – Consultant. Lilly – Consultant, other support, Speakers Bureau. Merck & Co., Inc., Rahway, NJ, USA – Consultant. Microba – Consultant. Mobius Care – Consultant. Morphic Therapeutics – Consultant. MRM Health – Consultant. Nexus Therapeutics – Consultant. Nimbus Discovery – Consultant. Odyssey Therapeutics – Consultant. Pfizer Inc – Consultant, Grant/Research Support, Other support, Speakers Bureau. Progenity – Consultant. Prometheus Biosciences – Consultant. Prometheus Laboratories – Consultant. Protagonist Therapeutics – Consultant. Q32 Bio – Consultant. Rasayana Therapeutics – Consultant. Recludix Therapeutics – Consultant. Reistone Biopharma – Consultant. Sanofi – Consultant. Spyre Therapeutics – Consultant. Sun Pharma – Consultant. Surrozen – Consultant. Takeda – Consultant, Grant/Research Support, Other support, Speakers Bureau. Target RWE – Consultant. Teva – Consultant. Theravance Biopharma – Consultant, Grant/Research Support. TLL Pharmaceutical – Consultant. Tr1X – Consultant. Union Therapeutics – Consultant. Ventyx Biopharma – Consultant, Stock Options, Stock-publicly held company(excluding mutual/index funds).
Taku Kobayashi: AbbVie – Advisory Committee/Board Member, Consultant, Grant/Research Support, Speakers Bureau. Alfresa Pharma – Advisory Committee/Board Member, Consultant, Grant/Research Support, Speakers Bureau. Alimentiv – Advisory Committee/Board Member, Consultant, Speakers Bureau. Astellas – Advisory Committee/Board Member, Consultant, Speakers Bureau. Bristol Myers Squibb – Advisory Committee/Board Member, Consultant, Speakers Bureau. Celltrion – Advisory Committee/Board Member, Consultant, Speakers Bureau. Covidien – Advisory Committee/Board Member, Consultant, Speakers Bureau. EA Pharma – Advisory Committee/Board Member, Consultant, Grant/Research Support, Speakers Bureau. Eli Lilly – Advisory Committee/Board Member, Consultant, Speakers Bureau. Ferring – Advisory Committee/Board Member, Consultant, Speakers Bureau. Galapagos – Advisory Committee/Board Member, Consultant, Speakers Bureau. Gilead Sciences – Advisory Committee/Board Member, Consultant, Grant/Research Support, Speakers Bureau. Janssen – Advisory Committee/Board Member, Consultant, Speakers Bureau. JIMRO – Advisory Committee/Board Member, Consultant, Speakers Bureau. JMDC – Advisory Committee/Board Member, Consultant, Speakers Bureau. Kissei Pharmaceutical – Advisory Committee/Board Member, Consultant, Speakers Bureau. Kyorin Pharmaceutical – Advisory Committee/Board Member, Consultant, Grant/Research Support, Speakers Bureau. Mitsubishi Tanabe Pharma – Advisory Committee/Board Member, Consultant, Speakers Bureau. Mochida Pharmaceutical – Advisory Committee/Board Member, Consultant, Grant/Research Support, Speakers Bureau. Nippon Kayaku – Advisory Committee/Board Member, Consultant, Grant/Research Support, Speakers Bureau. Otsuka Holdings – Grant/Research Support. Pfizer – Advisory Committee/Board Member, Consultant, Grant/Research Support, Speakers Bureau. Sekisui Medical – Grant/Research Support. Takeda – Advisory Committee/Board Member, Consultant, Grant/Research Support, Speakers Bureau. ThermoFisher Scientific – Advisory Committee/Board Member, Consultant, Grant/Research Support, Speakers Bureau. Zeria Pharmaceutical – Advisory Committee/Board Member, Consultant, Grant/Research Support, Speakers Bureau.
Jianmin Wu: Eli Lilly and Company – Employee, Stock Options.
Isabella Yali Wang: Eli Lilly and Company – Employee, Stock Options.
Baojin Zhu: Eli Lilly and Company – Employee, Stock Options.
Isabel Redondo: ELLI LILLY – Employee, Stock Options.
Laurent Peyrin-Biroulet: AbbVie – Grant/Research Support, Personal fees. Allergan – Personal Fees. Alma Bio Therapeutics – Personal Fees. Amgen – Personal Fees. Applied Molecular Transport – Personal Fees. Arena – Personal Fees. Biogen – Personal Fees. Boehringer Ingelheim – Personal Fees. Bristol Myers Squibb – Personal Fees. Celgene – Personal Fees. Celltrion – Personal Fees. CTMA – Stock Options. Enterome – Personal Fees. Enthera – Personal Fees. Ferring – Personal Fees. Fresenius Kabi – Personal Fees. Genentech – Personal Fees. Gilead – Personal Fees. Hikma – Personal Fees. InDex Pharmaceuticals – Personal Fees. Janssen – Personal Fees. Lilly – Personal Fees. MSD – Grant/Research Support, Personal Fees. Mylan – Personal Fees. Nestlé – Personal Fees. Norgine – Personal Fees. Oppilan Pharma – Personal Fees. OSE Immunotherapeutics – Personal Fees. Pfizer Inc – Personal Fees. Pharmacosmos – Fees. Samsung Bioepis – Personal Fees. Sandoz – Personal Fees. Sterna – Personal Fees. Sublimity Therapeutics – Personal Fees. Takeda – Grant/Research Support, Personal Fees. Tillotts – Personal Fees. Vifor – Personal Fees.
Alissa Walsh: AbbVie – Consultant, Grant/Research Support. Bühlmann Laboratories – Consultant, Grant/Research Support. Eli Lilly – Consultant, Grant/Research Support. Galapagos – Consultant, Grant/Research Support. GlaxoSmithKline – Consultant, Grant/Research Support. Janssen – Consultant, Grant/Research Support. Pfizer Inc – Consultant, Grant/Research Support. Takeda – Consultant, Grant/Research Support. Vifor – Consultant, Grant/Research Support.
Fernando Magro indicated no relevant financial relationships.
Bruce E.. Sands, MD, FACG1, Taku Kobayashi, MD2, Jianmin Wu, 3, Isabella Yali Wang, 3, Baojin Zhu, 3, Isabel Redondo, MD4, Laurent Peyrin-Biroulet, MD, PhD5, Alissa Walsh, MD6, Fernando Magro, MD, PhD7. P4354 - Long-Term Endoscopic and Histological Outcomes of Mirikizumab in Patients With Moderately to Severely Active Ulcerative Colitis With up to 3 Years of Treatment, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
