Tuesday Poster Session
Category: IBD
P4356 - Efficacy and Safety Profile of Etrasimod Was Not Impacted by Baseline Disease Activity: A Post Hoc Analysis of the ELEVATE UC Program
Tuesday, October 29, 2024
10:30 AM - 4:00 PM ET
Location: Exhibit Hall E
Has Audio

Bruce E. Sands, MD, FACG
Icahn School of Medicine at Mount Sinai
New York, NY
Presenting Author(s)
Bruce E.. Sands, MD, FACG1, Marla C. Dubinsky, MD1, Paulo G. Kotze, MD, MSc, PhD2, Séverine Vermeire, MD, PhD3, Remo Panaccione, MD4, Millie D. Long, MD, MPH5, John C. Woolcott, PhD6, Joseph Wu, PhD7, Aoibhinn McDonnell, PhD8, Martina Goetsch, MD9, Eustratios Bananis, PhD6, Andres J. Yarur, MD10
1Icahn School of Medicine at Mount Sinai, New York, NY; 2IBD Outpatient Clinics, Colorectal Surgery Unit, Pontifícia Universidade Católica do Paraná (PUCPR), Prado Velho, Curitiba, Parana, Brazil; 3University Hospitals Leuven, Leuven, Vlaams-Brabant, Belgium; 4University of Calgary, Calgary, AB, Canada; 5Center for Gastrointestinal Biology and Disease, University of North Carolina, Chapel Hill, NC; 6Pfizer Inc., Collegeville, PA; 7Pfizer Inc., Cambridge, MA; 8Pfizer Ltd., Sandwich, England, United Kingdom; 9Pfizer AG, Zürich, Zurich, Switzerland; 10Inflammatory Bowel Disease Center, Cedars-Sinai Medical Center, Los Angeles, CA
Introduction: Etrasimod is an oral, once-daily, selective sphingosine 1-phosphate (S1P)1,4,5 receptor modulator for the treatment of moderately to severely active ulcerative colitis (UC).
Methods: In this post hoc analysis of ELEVATE UC 52 (NCT03945188; treat-through design with 12-week [wk] induction then 40-wk maintenance periods) and ELEVATE UC 12 (NCT03996369; 12-wk induction period), etrasimod efficacy and safety were assessed according to baseline (BL) disease activity. Patients (pts) aged 16–80 years with moderately to severely active UC were randomized 2:1 to etrasimod 2 mg once daily or placebo (PBO).1 Pts’ BL UC activity was stratified as moderately or severely active based on a modified Mayo score (MMS) of 5–7 or 8–9, respectively. Efficacy endpoints including mean percentage change from BL in MMS and clinical response/remission per MMS category were measured at Wk12. Pooled efficacy data were analyzed descriptively; 95% confidence intervals (CIs) were calculated for all means or proportions with normal approximation or simultaneous estimation method, respectively. Safety was assessed up to Wk52 in the pooled safety analysis set.
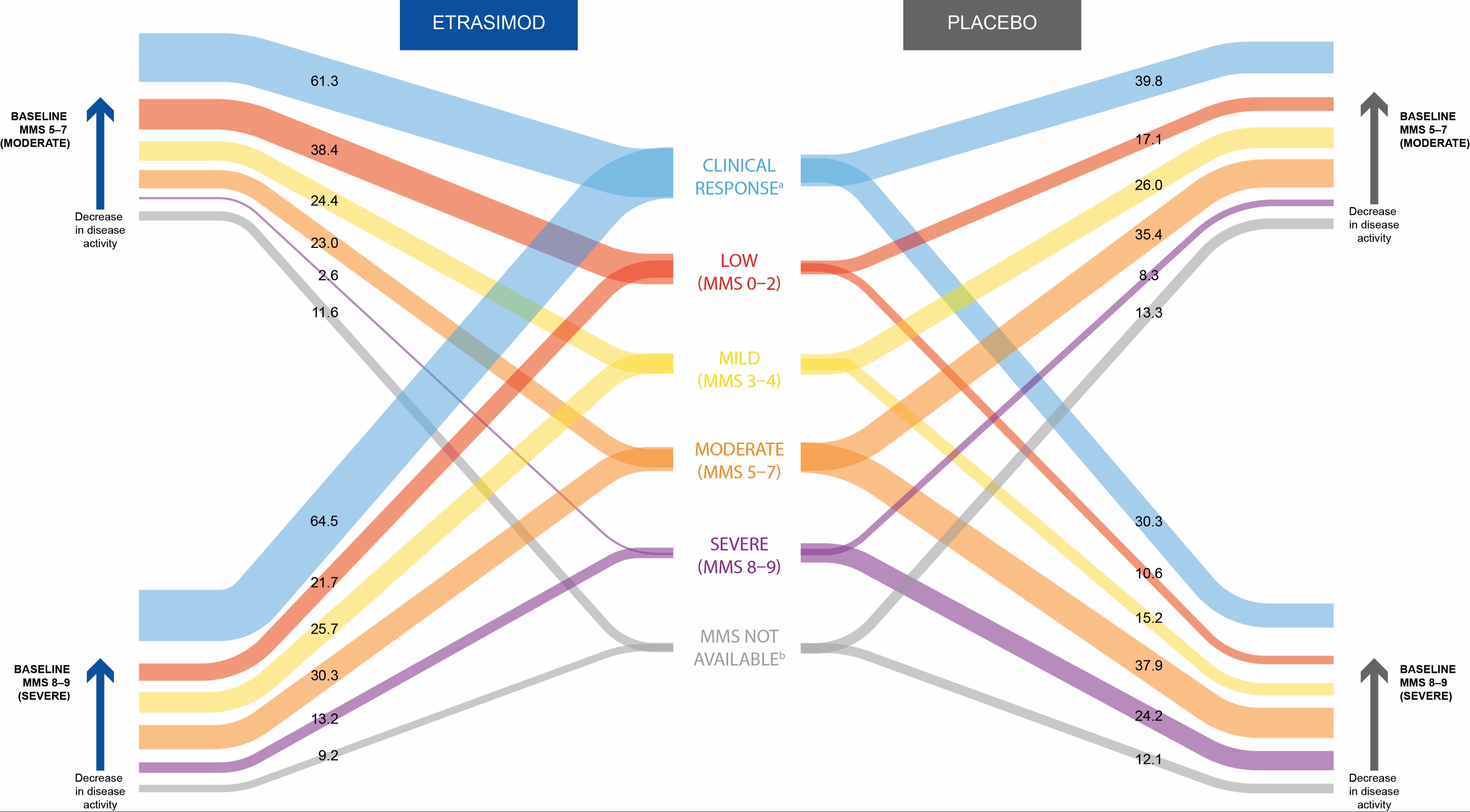
Results: Of the 743 pts in this analysis, 525 (70.7%) and 218 (29.3%) had a BL MMS of 5–7 and 8–9, respectively. BL characteristics were broadly similar across subgroups, except for lower rates of proctitis in pts with MMS 8–9 vs 5–7 (3.3% and 3.0% vs 9.3% and 9.4% in etrasimod and PBO groups, respectively; Table). At Wk12, pts taking etrasimod had larger mean percentage reductions (95% CI) in MMS vs PBO, regardless of BL disease activity (48.4% [52.3, 44.4] vs 27.0% [32.2, 21.7] for BL MMS 5–7, and 46.4% [51.2, 41.5] vs 29.8% [37.2, 22.3] for BL MMS 8–9). At Wk12, more pts taking etrasimod achieved clinical response vs PBO, regardless of BL disease activity, with an overall reduction from BL disease activity as measured by MMS categorization in both BL MMS subgroups (Figure). Proportions of pts with treatment-emergent adverse events (TEAEs) were broadly similar across treatment and BL disease activity subgroups (any TEAE: 59.0% vs 50.8% and 65.1% vs 56.1% for etrasimod vs PBO, in BL MMS 5–7 and 8–9 groups, respectively).
Discussion: Etrasimod showed greater reductions in disease severity and higher rates of clinical response, vs PBO, regardless of BL disease activity. Its safety profile was consistent with the overall trial population1 and was not impacted by BL disease activity.
Reference:
1. Sandborn WJ et al. Lancet 2023; 401: 1159–1171.
Note: The table for this abstract can be viewed in the ePoster Gallery section of the ACG 2024 ePoster Site or in The American Journal of Gastroenterology's abstract supplement issue, both of which will be available starting October 27, 2024.
Disclosures:
Bruce E.. Sands, MD, FACG1, Marla C. Dubinsky, MD1, Paulo G. Kotze, MD, MSc, PhD2, Séverine Vermeire, MD, PhD3, Remo Panaccione, MD4, Millie D. Long, MD, MPH5, John C. Woolcott, PhD6, Joseph Wu, PhD7, Aoibhinn McDonnell, PhD8, Martina Goetsch, MD9, Eustratios Bananis, PhD6, Andres J. Yarur, MD10. P4356 - Efficacy and Safety Profile of Etrasimod Was Not Impacted by Baseline Disease Activity: A Post Hoc Analysis of the ELEVATE UC Program, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
1Icahn School of Medicine at Mount Sinai, New York, NY; 2IBD Outpatient Clinics, Colorectal Surgery Unit, Pontifícia Universidade Católica do Paraná (PUCPR), Prado Velho, Curitiba, Parana, Brazil; 3University Hospitals Leuven, Leuven, Vlaams-Brabant, Belgium; 4University of Calgary, Calgary, AB, Canada; 5Center for Gastrointestinal Biology and Disease, University of North Carolina, Chapel Hill, NC; 6Pfizer Inc., Collegeville, PA; 7Pfizer Inc., Cambridge, MA; 8Pfizer Ltd., Sandwich, England, United Kingdom; 9Pfizer AG, Zürich, Zurich, Switzerland; 10Inflammatory Bowel Disease Center, Cedars-Sinai Medical Center, Los Angeles, CA
Introduction: Etrasimod is an oral, once-daily, selective sphingosine 1-phosphate (S1P)1,4,5 receptor modulator for the treatment of moderately to severely active ulcerative colitis (UC).
Methods: In this post hoc analysis of ELEVATE UC 52 (NCT03945188; treat-through design with 12-week [wk] induction then 40-wk maintenance periods) and ELEVATE UC 12 (NCT03996369; 12-wk induction period), etrasimod efficacy and safety were assessed according to baseline (BL) disease activity. Patients (pts) aged 16–80 years with moderately to severely active UC were randomized 2:1 to etrasimod 2 mg once daily or placebo (PBO).1 Pts’ BL UC activity was stratified as moderately or severely active based on a modified Mayo score (MMS) of 5–7 or 8–9, respectively. Efficacy endpoints including mean percentage change from BL in MMS and clinical response/remission per MMS category were measured at Wk12. Pooled efficacy data were analyzed descriptively; 95% confidence intervals (CIs) were calculated for all means or proportions with normal approximation or simultaneous estimation method, respectively. Safety was assessed up to Wk52 in the pooled safety analysis set.
Results: Of the 743 pts in this analysis, 525 (70.7%) and 218 (29.3%) had a BL MMS of 5–7 and 8–9, respectively. BL characteristics were broadly similar across subgroups, except for lower rates of proctitis in pts with MMS 8–9 vs 5–7 (3.3% and 3.0% vs 9.3% and 9.4% in etrasimod and PBO groups, respectively; Table). At Wk12, pts taking etrasimod had larger mean percentage reductions (95% CI) in MMS vs PBO, regardless of BL disease activity (48.4% [52.3, 44.4] vs 27.0% [32.2, 21.7] for BL MMS 5–7, and 46.4% [51.2, 41.5] vs 29.8% [37.2, 22.3] for BL MMS 8–9). At Wk12, more pts taking etrasimod achieved clinical response vs PBO, regardless of BL disease activity, with an overall reduction from BL disease activity as measured by MMS categorization in both BL MMS subgroups (Figure). Proportions of pts with treatment-emergent adverse events (TEAEs) were broadly similar across treatment and BL disease activity subgroups (any TEAE: 59.0% vs 50.8% and 65.1% vs 56.1% for etrasimod vs PBO, in BL MMS 5–7 and 8–9 groups, respectively).
Discussion: Etrasimod showed greater reductions in disease severity and higher rates of clinical response, vs PBO, regardless of BL disease activity. Its safety profile was consistent with the overall trial population1 and was not impacted by BL disease activity.
Reference:
1. Sandborn WJ et al. Lancet 2023; 401: 1159–1171.

Figure: Proportion of pts achieving clinical response in each MMS category at Wk12 stratified according to baseline disease activity (pooled data from ELEVATE UC 52 and ELEVATE UC 12).
[a]Clinical response was defined as a ≥ 2-point and ≥ 30% decrease from baseline in MMS, and a ≥ 1-point decrease from baseline in RBS, or RBS ≤ 1.
[b]MMS was not available for some pts at Wk12 for various reasons, including discontinuation prior to Wk12.
Data were pooled from ELEVATE UC 52 and ELEVATE UC 12.
MMS, modified Mayo score; pt, patient; RBS, rectal bleeding subscore; UC, ulcerative colitis; Wk, Week.
[a]Clinical response was defined as a ≥ 2-point and ≥ 30% decrease from baseline in MMS, and a ≥ 1-point decrease from baseline in RBS, or RBS ≤ 1.
[b]MMS was not available for some pts at Wk12 for various reasons, including discontinuation prior to Wk12.
Data were pooled from ELEVATE UC 52 and ELEVATE UC 12.
MMS, modified Mayo score; pt, patient; RBS, rectal bleeding subscore; UC, ulcerative colitis; Wk, Week.
Note: The table for this abstract can be viewed in the ePoster Gallery section of the ACG 2024 ePoster Site or in The American Journal of Gastroenterology's abstract supplement issue, both of which will be available starting October 27, 2024.
Disclosures:
Bruce Sands: AbbVie – Consultant. Abivax – Consultant, Speakers Bureau. Adiso Therapeutics – Consultant. Agomab – Consultant. Alimentiv – Consultant. Amgen – Consultant. AnaptysBio – Consultant. Arena Pharmaceuticals – Consultant. Artugen Therapeutics – Consultant. AstraZeneca – Consultant. Biolojic Design – Consultant. Biora Therapeutics – Consultant. Boehringer Ingelheim – Consultant. Boston Pharmaceuticals – Consultant. Bristol Myers Squibb – Consultant, Grant/Research Support, Other support, Speakers Bureau. Calibr – Consultant. Celgene – Consultant. Celltrion – Consultant. ClostraBio – Consultant. Enthera – Consultant. Envied Biosciences – Consultant. Equilium – Consultant. Evommune – Consultant. Ferring – Consultant. Fiat – Consultant. Fresenius Kabi – Consultant. Galapagos – Consultant. Genentech (Roche) – Consultant. Gilead Sciences – Consultant. Glaxo SmithKline – Consultant. Gossamer Bio – Consultant. Imhotex – Consultant. Index Pharmaceuticals – Consultant. Innovation Pharmaceuticals – Consultant. Inotrem – Consultant. Janssen – Consultant, Grant/Research Support, Other support, Speakers Bureau. Kaleido – Consultant. Kallyope – Consultant. Lilly – Consultant, other support, Speakers Bureau. Merck & Co., Inc., Rahway, NJ, USA – Consultant. Microba – Consultant. Mobius Care – Consultant. Morphic Therapeutics – Consultant. MRM Health – Consultant. Nexus Therapeutics – Consultant. Nimbus Discovery – Consultant. Odyssey Therapeutics – Consultant. Pfizer Inc – Consultant, Grant/Research Support, Other support, Speakers Bureau. Progenity – Consultant. Prometheus Biosciences – Consultant. Prometheus Laboratories – Consultant. Protagonist Therapeutics – Consultant. Q32 Bio – Consultant. Rasayana Therapeutics – Consultant. Recludix Therapeutics – Consultant. Reistone Biopharma – Consultant. Sanofi – Consultant. Spyre Therapeutics – Consultant. Sun Pharma – Consultant. Surrozen – Consultant. Takeda – Consultant, Grant/Research Support, Other support, Speakers Bureau. Target RWE – Consultant. Teva – Consultant. Theravance Biopharma – Consultant, Grant/Research Support. TLL Pharmaceutical – Consultant. Tr1X – Consultant. Union Therapeutics – Consultant. Ventyx Biopharma – Consultant, Stock Options, Stock-publicly held company(excluding mutual/index funds).
Marla Dubinsky: AbbVie – Consultant. Abivax – Consultant. Arena – Consultant. AstraZeneca – Consultant. Bristol Myers Squibb – Consultant. Celgene – Consultant. Eli Lilly – Consultant. Genentech – Consultant. Gilead – Consultant. Janssen – Consultant. Pfizer Inc – Consultant. Prometheus Labs – Consultant. Takeda – Consultant.
Paulo Kotze: AbbVie – Consultant, Speakers Bureau. Janssen – Consultant, Speakers Bureau. Pfizer Inc – Consultant, Grant/Research Support, Speakers Bureau. Takeda – Consultant, Grant/Research Support, Speakers Bureau.
Séverine Vermeire: AbbVie – Consultant, Grant/Research Support. Abivax – Consultant. AbolerlsPharma – Consultant. AgomAb – Consultant. Alimentiv – Consultant. Arena Pharmaceuticals – Consultant. AstraZeneca – Consultant. BioraTherapeutics – Consultant. Boehringer Ingelheim – Consultant. Bristol Myers Squibb – Consultant. Celgene – Consultant. Cytoki Pharma – Consultant. Dr Falk Pharma – Consultant. Eli Lilly – Consultant. Ferring – Consultant. Galapagos – Consultant, Grant/Research Support. Genentech Roche – Consultant. Gilead – Consultant. GSK – Consultant. Hospira – Consultant. J&J – Consultant, Grant/Research Support. Janssen – Consultant. lmidomics – Consultant. Materia Prima – Consultant. Mestag Therapeutics – Consultant. Microbiotica – Consultant. MiroBio – Consultant. Morphic – Consultant. MrMHealth – Consultant. MSD – Consultant. Mundipharma – Consultant. Pfizer Inc – Consultant, Grant/Research Support. Prodigest – Consultant. Progenity – Consultant. Prometheus – Consultant. Robarts Clinical Trials – Consultant. Surrozen – Consultant. Takeda – Consultant, Grant/Research Support. Theravance – Consultant. Tillots Pharma AG – Consultant. VectivBio – Consultant. Ventyx – Consultant. Zealand Pharma – Consultant.
Remo Panaccione: Élan – Consultant. Abbivax – Consultant. Abbott – Consultant. AbbVie – Advisory Committee/Board Member, Consultant, Speaking Fees. Alimentiv – Advisory Committee/Board Member, Consultant. Amgen – Advisory Committee/Board Member, Consultant, Speaker Fees. Arena Pharmaceuticals – Advisory Committee/Board Member, Consultant, Speaker Fees. AstraZeneca – Advisory Committee/Board Member, Consultant. Biogen – Advisory Committee/Board Member, Consultant. Boehringer Ingelheim – Advisory Committee/Board Member, Consultant. Bristol Myers Squibb – Advisory Committee/Board Member, Consultant, Speaker Fees. Celgene – Advisory Committee/Board Member, Consultant, Speaker Fees. Celltrion – Consultant. Cosmos Pharmaceuticals – Consultant. Eisai – Consultant. Ferring – Advisory Committee/Board Member, Consultant, Speaker Fees. Fresenius Kabi – Advisory Committee/Board Member, Consultant, Speaker Fees. Galapagos – Consultant. Genentech – Advisory Committee/Board Member, Consultant. Gilead – Advisory Committee/Board Member, Consultant, Speaker Fees. GlaxoSmithKline – Advisory Committee/Board Member, Consultant. JAMP Bio – Advisory Committee/Board Member, Consultant. Janssen – Advisory Committee/Board Member, Consultant, Speaker Fees. Lilly – Advisory Committee/Board Member, Consultant, Speaker Fees. Merck – Advisory Committee/Board Member, Consultant, Speaker Fees. Mylan – Advisory Committee/Board Member, Consultant. Novartis – Advisory Committee/Board Member, Consultant. Oppilan Pharma – Advisory Committee/Board Member, Consultant. Organon – Advisory Committee/Board Member, Consultant, Speaker Fees. Pandion Pharma – Advisory Committee/Board Member, Consultant. Pendopharm – Consultant. Pfizer Inc – Advisory Committee/Board Member, Consultant, Speaker Fees. Progenity – Advisory Committee/Board Member, Consultant. Prometheus Biosciences – Consultant. Protagonist Therapeutics – Advisory Committee/Board Member, Consultant. Roche – Advisory Committee/Board Member, Consultant, Speaker Fees. Sandoz – Advisory Committee/Board Member, Consultant, Speaker Fees. Satisfai Health – Consultant. Shire – Advisory Committee/Board Member, Consultant, Speaker Fees. Sublimity Therapeutics – Advisory Committee/Board Member, Consultant. Takeda – Advisory Committee/Board Member, Consultant, Speaker Fees. Theravance – Consultant. Trellus – Consultant. UCB – Consultant. Ventyx – Advisory Committee/Board Member, Consultant. Viatris – Consultant.
Millie Long: AbbVie – Consultant. Bristol-Myers Squibb – Consultant. Eli Lilly – Consultant, Grant/Research Support. Intercept – Consultant. Janssen – Consultant. Pfizer Inc – Consultant, Grant/Research Support. Prometheus – Consultant. Roche – Consultant. Roivant – Consultant. Takeda – Consultant, Grant/Research Support. Target RWE – Consultant.
John Woolcott: Pfizer Inc – Employee, Stock Options.
Joseph Wu: Pfizer Inc – Employee, Stock Options.
Aoibhinn McDonnell: Pfizer Inc – Stock Options. Pfizer Ltd – Employee.
Martina Goetsch: Pfizer AG – Employee, Stock Options.
Eustratios Bananis: Pfizer Inc. – Employee, Stock Options.
Andres Yarur: AbbVie – Consultant, Lecture Fees. Arena – Consultant. Bristol Myers Squibb – Consultant, Lecture Fees. Pfizer Inc – Consultant. Takeda – Consultant.
Bruce E.. Sands, MD, FACG1, Marla C. Dubinsky, MD1, Paulo G. Kotze, MD, MSc, PhD2, Séverine Vermeire, MD, PhD3, Remo Panaccione, MD4, Millie D. Long, MD, MPH5, John C. Woolcott, PhD6, Joseph Wu, PhD7, Aoibhinn McDonnell, PhD8, Martina Goetsch, MD9, Eustratios Bananis, PhD6, Andres J. Yarur, MD10. P4356 - Efficacy and Safety Profile of Etrasimod Was Not Impacted by Baseline Disease Activity: A Post Hoc Analysis of the ELEVATE UC Program, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
