Tuesday Poster Session
Category: IBD
P4375 - Matching-Adjusted Indirect Comparison of Etrasimod Versus Ozanimod for Clinical Response and Remission Among Patients With Moderately to Severely Active Ulcerative Colitis
Tuesday, October 29, 2024
10:30 AM - 4:00 PM ET
Location: Exhibit Hall E
Has Audio
- PH
Peter Hur, PharmD
Pfizer Inc.
New York, NY
Presenting Author(s)
Vipul Jairath, MBChB1, Thomas P. Leahy, PhD2, Ravi Potluri, PhD3, Karolina Wosik, MSc, PhD4, David Gruben, PhD5, Joseph C. Cappelleri, PhD, MPH5, Peter Hur, PharmD6, Lauren Bartolome, PharmD, MS6
1Western University, London, ON, Canada; 2Putnam Associates, Toronto, ON, Canada; 3Putnam Associates, New York, NY; 4Pfizer Canada, Kirkland, PQ, Canada; 5Pfizer Inc., Groton, CT; 6Pfizer Inc., New York, NY
Introduction: Etrasimod (ETR) and ozanimod (OZN) are selective sphingosine 1-phosphate receptor modulators targeting the S1P1,4,5, and S1P 1,5 receptors, respectively, for the treatment of moderately to severely active ulcerative colitis (UC). While network meta-analyses have been conducted that included both ETR and OZN, differences in populations and trial designs can impact outcomes. The aim was to conduct matching-adjusted indirect comparisons (MAIC) between ETR and OZN to compare clinical response and remission after the induction period and among induction phase responders following the maintenance period.
Methods: Data from phase 3 clinical trials of ETR and OZN that reported data on both induction and maintenance were included in the analyses, namely ELEVATE UC 52 and TRUE NORTH. Patients (pts) in ELEVATE UC 52 received 2mg ETR or placebo (PBO) throughout induction and maintenance, in a treat-through trial design, while pts in TRUE NORTH received 1mg equivalent dose of OZN or PBO during induction, OZN pts with clinical response after induction were further rerandomized to PBO or OZN for maintenance. A PBO anchored approach was used for induction results, while an unanchored approach was conducted for maintenance due to differences in the maintenance PBO arms resulting from the distinct trial designs. Patient level data from ELEVATE UC 52 were weighted using MAIC methodology to match the TRUE NORTH population. Pts were matched on age, sex, corticosteroid use, duration of UC, previous biologic exposure, modified Mayo Score, and presence of left-sided disease, all measured at baseline.
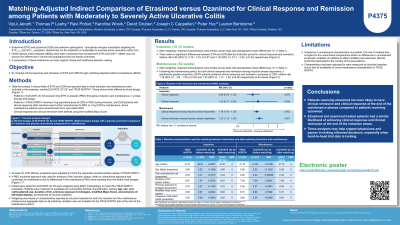
Results: After MAIC weighting, matched characteristics were similar with standardized mean differences < 0.1. There were no significant differences between ETR and OZN after the induction period for the outcomes of clinical response and remission (Table1). However, following the maintenance period, for both clinical response and remission among induction phase responders, a significantly greater proportion of ETR pts achieved clinical response and remission compared to OZN (relative risk 1.19 (95% CI, 1.06-1.31), p< 0.05 and 1.34 (95% CI, 1.11-1.55), p< 0.05 respectively) at 52 weeks.
Discussion: MAIC results suggest superiority of ETR over OZN in terms of clinical response and remission at the end of the maintenance period (52 weeks), while both treatments are similar after the induction period (10-12 weeks). Additional comparisons of other efficacy and safety outcomes will further facilitate treatment decisions.
Note: The table for this abstract can be viewed in the ePoster Gallery section of the ACG 2024 ePoster Site or in The American Journal of Gastroenterology's abstract supplement issue, both of which will be available starting October 27, 2024.
Disclosures:
Vipul Jairath, MBChB1, Thomas P. Leahy, PhD2, Ravi Potluri, PhD3, Karolina Wosik, MSc, PhD4, David Gruben, PhD5, Joseph C. Cappelleri, PhD, MPH5, Peter Hur, PharmD6, Lauren Bartolome, PharmD, MS6. P4375 - Matching-Adjusted Indirect Comparison of Etrasimod Versus Ozanimod for Clinical Response and Remission Among Patients With Moderately to Severely Active Ulcerative Colitis, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
1Western University, London, ON, Canada; 2Putnam Associates, Toronto, ON, Canada; 3Putnam Associates, New York, NY; 4Pfizer Canada, Kirkland, PQ, Canada; 5Pfizer Inc., Groton, CT; 6Pfizer Inc., New York, NY
Introduction: Etrasimod (ETR) and ozanimod (OZN) are selective sphingosine 1-phosphate receptor modulators targeting the S1P1,4,5, and S1P 1,5 receptors, respectively, for the treatment of moderately to severely active ulcerative colitis (UC). While network meta-analyses have been conducted that included both ETR and OZN, differences in populations and trial designs can impact outcomes. The aim was to conduct matching-adjusted indirect comparisons (MAIC) between ETR and OZN to compare clinical response and remission after the induction period and among induction phase responders following the maintenance period.
Methods: Data from phase 3 clinical trials of ETR and OZN that reported data on both induction and maintenance were included in the analyses, namely ELEVATE UC 52 and TRUE NORTH. Patients (pts) in ELEVATE UC 52 received 2mg ETR or placebo (PBO) throughout induction and maintenance, in a treat-through trial design, while pts in TRUE NORTH received 1mg equivalent dose of OZN or PBO during induction, OZN pts with clinical response after induction were further rerandomized to PBO or OZN for maintenance. A PBO anchored approach was used for induction results, while an unanchored approach was conducted for maintenance due to differences in the maintenance PBO arms resulting from the distinct trial designs. Patient level data from ELEVATE UC 52 were weighted using MAIC methodology to match the TRUE NORTH population. Pts were matched on age, sex, corticosteroid use, duration of UC, previous biologic exposure, modified Mayo Score, and presence of left-sided disease, all measured at baseline.
Results: After MAIC weighting, matched characteristics were similar with standardized mean differences < 0.1. There were no significant differences between ETR and OZN after the induction period for the outcomes of clinical response and remission (Table1). However, following the maintenance period, for both clinical response and remission among induction phase responders, a significantly greater proportion of ETR pts achieved clinical response and remission compared to OZN (relative risk 1.19 (95% CI, 1.06-1.31), p< 0.05 and 1.34 (95% CI, 1.11-1.55), p< 0.05 respectively) at 52 weeks.
Discussion: MAIC results suggest superiority of ETR over OZN in terms of clinical response and remission at the end of the maintenance period (52 weeks), while both treatments are similar after the induction period (10-12 weeks). Additional comparisons of other efficacy and safety outcomes will further facilitate treatment decisions.
Note: The table for this abstract can be viewed in the ePoster Gallery section of the ACG 2024 ePoster Site or in The American Journal of Gastroenterology's abstract supplement issue, both of which will be available starting October 27, 2024.
Disclosures:
Vipul Jairath: AbbVie – Consultant, Grant/Research Support, Speakers Bureau. Alimentiv – Consultant, Employee, Grant/Research Support, Speakers Bureau. Arena Pharmaceuticals – Consultant, Grant/Research Support, Speakers Bureau. Asahi Kasei Pharma – Consultant, Grant/Research Support, Speakers Bureau. Asieris Pharmaceuticals – Consultant, Grant/Research Support, Speakers Bureau. AstraZeneca – Consultant, Grant/Research Support, Speakers Bureau. Avoro Capital – Advisory Committee/Board Member, Consultant. Bristol Myers Squibb – Consultant, Grant/Research Support, Speakers Bureau. Celltrion – Consultant, Grant/Research Support, Speakers Bureau. Eli Lilly and Company – Consultant, Grant/Research Support, Speakers Bureau. Endpoint Health – Advisory Committee/Board Member, Consultant. Enthera – Advisory Committee/Board Member, Consultant. Ferring Pharmaceuticals – Consultant, Grant/Research Support, Speakers Bureau. Flagship Pioneering – Consultant, Grant/Research Support, Speakers Bureau. Fresenius Kabi – Consultant, Grant/Research Support, Speakers Bureau. Galapagos NV – Consultant, Grant/Research Support, Speakers Bureau. Genentech – Consultant, Grant/Research Support, Speakers Bureau. Gilde Healthcare – Advisory Committee/Board Member, Consultant. Gilead Sciences – Consultant, Grant/Research Support, Speakers Bureau. GlaxoSmithKline – Consultant, Grant/Research Support, Speakers Bureau. Innomar – Advisory Committee/Board Member, Consultant. JAMP – Advisory Committee/Board Member, Consultant. Janssen – Consultant, Grant/Research Support, Speakers Bureau. London Health Sciences Centre – Employee. Merck – Consultant, Grant/Research Support, Speakers Bureau. Metacrine – Consultant, Grant/Research Support, Speakers Bureau. Mylan – Consultant, Grant/Research Support, Speakers Bureau. Pandion Therapeutics – Consultant, Grant/Research Support, Speakers Bureau. Pendopharm – Consultant, Grant/Research Support, Speakers Bureau. Pfizer – Consultant, Grant/Research Support, Speakers Bureau. Prometheus Therapeutics and Diagnostics – Consultant, Grant/Research Support, Speakers Bureau. Protagonist Therapeutics – Consultant, Grant/Research Support, Speakers Bureau. Reistone Biopharma – Consultant, Grant/Research Support, Speakers Bureau. Roche – Consultant, Grant/Research Support, Speakers Bureau. Roivant – Advisory Committee/Board Member, Consultant. Sandoz – Consultant, Grant/Research Support, Speakers Bureau. SCOPE – Advisory Committee/Board Member, Consultant. Second Genome – Consultant, Grant/Research Support, Speakers Bureau. Shire – Speakers Bureau. Sorriso Pharmaceuticals – Consultant, Grant/Research Support, Speakers Bureau. Synedgen – Advisory Committee/Board Member, Consultant. Takeda – Consultant, Grant/Research Support, Speakers Bureau. TD Securities – Advisory Committee/Board Member, Consultant. Teva – Consultant, Grant/Research Support, Speakers Bureau. Topivert – Consultant, Grant/Research Support, Speakers Bureau. Ventyx Biosciences – Consultant, Grant/Research Support, Speakers Bureau. Vividion Therapeutics – Consultant, Grant/Research Support, Speakers Bureau.
Thomas Leahy: Angelini – Consultant. Immunocore – Consultant. Pfizer – Consultant, Stock-publicly held company(excluding mutual/index funds). Rigel Pharmaceuticals – Consultant. Roche – Consultant.
Ravi Potluri: AstraZeneca – Consultant. Bristol Myers Squibb – Consultant. J&J – Consultant. Pfizer – Consultant. Servier – Consultant.
Karolina Wosik: Pfizer Canada Inc – Employee, Stock Options.
David Gruben: Pfizer Inc – Employee, Stock Options.
Joseph Cappelleri: Pfizer Inc – Employee, Stock Options.
Peter Hur: Clene Nanomedicine – Stock Options. Haleon – Stock Options. Isdoria – Stock Options. Liquidia – Stock Options. Longboard Pharmaceuticals – Stock Options. Pfizer Inc – Employee, Stock Options. Proctor Gamble – Stock Options. US 2022/0257594 A1 – Intellectual Property/Patents.
Lauren Bartolome: Pfizer – Employee, Stock Options, Stock-publicly held company(excluding mutual/index funds).
Vipul Jairath, MBChB1, Thomas P. Leahy, PhD2, Ravi Potluri, PhD3, Karolina Wosik, MSc, PhD4, David Gruben, PhD5, Joseph C. Cappelleri, PhD, MPH5, Peter Hur, PharmD6, Lauren Bartolome, PharmD, MS6. P4375 - Matching-Adjusted Indirect Comparison of Etrasimod Versus Ozanimod for Clinical Response and Remission Among Patients With Moderately to Severely Active Ulcerative Colitis, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
