Tuesday Poster Session
Category: IBD
P4380 - Burden and Impact of Fatigue on Patients with Crohn’s Disease and Ulcerative Colitis in the United States and Europe: Results from the Communicating Needs and Features of IBD Experiences (CONFIDE) Survey
Tuesday, October 29, 2024
10:30 AM - 4:00 PM ET
Location: Exhibit Hall E

Has Audio

Remo Panaccione, MD
University of Calgary
Calgary, AB, Canada
Presenting Author(s)
Remo Panaccione, MD1, Alison Potts Bleakman, PhD2, Stefan Schreiber, MD3, Simon Travis, MBBS, MRCP, PhD4, Marla C. Dubinsky, MD5, Toshifumi Hibi, MD, PhD6, Theresa Gibble, PhD2, Tommaso Panni, PhD2, Cem Kayhan, MD2, Eoin Flynn, PhD2, Angelo Favia, PhD2, Christian Atkinson, MRes7, David T.. Rubin, MD, FACG8
1University of Calgary, Calgary, AB, Canada; 2Eli Lilly and Company, Indianapolis, IN; 3University Hospital, Kiel, Schleswig-Holstein, Germany; 4University of Oxford, Oxford, England, United Kingdom; 5Icahn School of Medicine at Mount Sinai, New York, NY; 6Kitasato University, Tokyo, Japan, Shirokane, Minato-ku, Tokyo, Japan; 7Adelphi Real World, Bollington, England, United Kingdom; 8University of Chicago Medicine, Inflammatory Bowel Disease Center, Chicago, IL
Introduction: Fatigue is a common yet debilitating symptom with multifactorial etiology (e.g., inflammation, sleep disturbances) which significantly impacts quality of life of patients (pts) with inflammatory bowel disease (IBD). The Communicating Needs and Features of IBD Experiences (CONFIDE) study explored the burden and impact of Crohn’s disease/ulcerative colitis (CD/UC)-related symptoms on pts’ lives in the United States (US), Europe (EU5: France, Germany, Italy, Spain, and UK) and Japan.
Methods: An online, quantitative, cross-sectional survey was conducted in pts with moderate-to-severe CD/UC. Moderate-to-severe CD/UC were defined using criteria based on previous treatment, steroid use, and/or hospitalization. Patients were asked about the impact of CD/UC-related fatigue on their work/school, social, physical, and sexual activities. Descriptive statistics were used to summarize data from US and EU5.
Results: Overall, 215 US (males [M]=55%, mean age=41 years) and 547 EU5 (M=55%, mean age=38 years) pts with CD, and 200 US (M=62%, mean age=40 years) and 556 EU5 (M=57%, mean age=39 years) pts with UC completed the survey (Table 1).
Overall, 36% US and 34% EU5 pts with CD, and 28% US and 21% EU5 pts with UC reported currently experiencing fatigue (in the past month). A total of 46% US and 45% EU5 pts with CD, and 40% US and 31% EU5 pts with UC reported ever experiencing fatigue. Similar results were observed in pts receiving advanced therapies (Current: CD, US: 36%, EU5: 34%; UC, US: 26%, EU5: 20%; Ever: CD, US: 47%, EU5: 46%; UC, US: 36%, EU5: 29%).
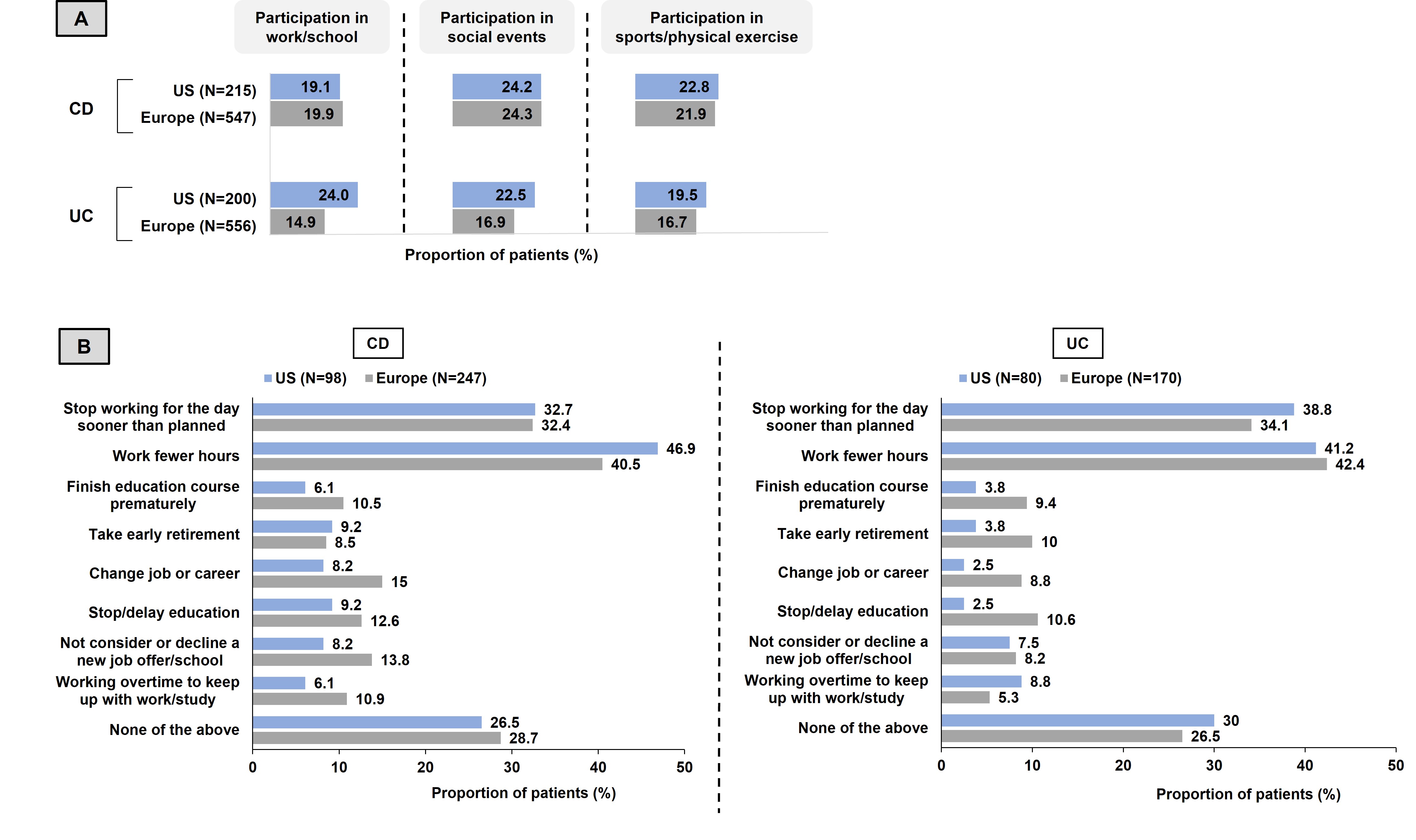
In past 3 months, 19–24% and 15–24% pts with CD and UC, respectively reported declining participation in work/school, social activities, and sports/physical exercise due to fatigue (Fig 1A). Among pts with CD/UC who ever experienced fatigue, most reported that fatigue impacted their work/school-related activities (CD, US:74%, EU5:71%; UC, US:70%, EU5:74%). Of these, >40% pts worked for fewer hours and >30% stopped working for the day sooner than planned due to CD/UC-related fatigue (Fig 1B).
Among pts who reported reduced sexual activity in past 3 months (CD, US:69%, EU5:56%; UC, US:63%, EU5:53%), 38% US and 30% EU5 pts with CD, and 29% US and 18% EU5 pts with UC attributed it to CD/UC-related fatigue.
Discussion: Fatigue is a prevalent symptom of moderate-to-severe CD/UC which adversely affects pts’ work/school, social, physical, and sexual activities. This underscores the need to develop fatigue-related treatment strategies in CD and UC.
Note: The table for this abstract can be viewed in the ePoster Gallery section of the ACG 2024 ePoster Site or in The American Journal of Gastroenterology's abstract supplement issue, both of which will be available starting October 27, 2024.
Disclosures:
Remo Panaccione, MD1, Alison Potts Bleakman, PhD2, Stefan Schreiber, MD3, Simon Travis, MBBS, MRCP, PhD4, Marla C. Dubinsky, MD5, Toshifumi Hibi, MD, PhD6, Theresa Gibble, PhD2, Tommaso Panni, PhD2, Cem Kayhan, MD2, Eoin Flynn, PhD2, Angelo Favia, PhD2, Christian Atkinson, MRes7, David T.. Rubin, MD, FACG8. P4380 - Burden and Impact of Fatigue on Patients with Crohn’s Disease and Ulcerative Colitis in the United States and Europe: Results from the Communicating Needs and Features of IBD Experiences (CONFIDE) Survey, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
1University of Calgary, Calgary, AB, Canada; 2Eli Lilly and Company, Indianapolis, IN; 3University Hospital, Kiel, Schleswig-Holstein, Germany; 4University of Oxford, Oxford, England, United Kingdom; 5Icahn School of Medicine at Mount Sinai, New York, NY; 6Kitasato University, Tokyo, Japan, Shirokane, Minato-ku, Tokyo, Japan; 7Adelphi Real World, Bollington, England, United Kingdom; 8University of Chicago Medicine, Inflammatory Bowel Disease Center, Chicago, IL
Introduction: Fatigue is a common yet debilitating symptom with multifactorial etiology (e.g., inflammation, sleep disturbances) which significantly impacts quality of life of patients (pts) with inflammatory bowel disease (IBD). The Communicating Needs and Features of IBD Experiences (CONFIDE) study explored the burden and impact of Crohn’s disease/ulcerative colitis (CD/UC)-related symptoms on pts’ lives in the United States (US), Europe (EU5: France, Germany, Italy, Spain, and UK) and Japan.
Methods: An online, quantitative, cross-sectional survey was conducted in pts with moderate-to-severe CD/UC. Moderate-to-severe CD/UC were defined using criteria based on previous treatment, steroid use, and/or hospitalization. Patients were asked about the impact of CD/UC-related fatigue on their work/school, social, physical, and sexual activities. Descriptive statistics were used to summarize data from US and EU5.
Results: Overall, 215 US (males [M]=55%, mean age=41 years) and 547 EU5 (M=55%, mean age=38 years) pts with CD, and 200 US (M=62%, mean age=40 years) and 556 EU5 (M=57%, mean age=39 years) pts with UC completed the survey (Table 1).
Overall, 36% US and 34% EU5 pts with CD, and 28% US and 21% EU5 pts with UC reported currently experiencing fatigue (in the past month). A total of 46% US and 45% EU5 pts with CD, and 40% US and 31% EU5 pts with UC reported ever experiencing fatigue. Similar results were observed in pts receiving advanced therapies (Current: CD, US: 36%, EU5: 34%; UC, US: 26%, EU5: 20%; Ever: CD, US: 47%, EU5: 46%; UC, US: 36%, EU5: 29%).
In past 3 months, 19–24% and 15–24% pts with CD and UC, respectively reported declining participation in work/school, social activities, and sports/physical exercise due to fatigue (Fig 1A). Among pts with CD/UC who ever experienced fatigue, most reported that fatigue impacted their work/school-related activities (CD, US:74%, EU5:71%; UC, US:70%, EU5:74%). Of these, >40% pts worked for fewer hours and >30% stopped working for the day sooner than planned due to CD/UC-related fatigue (Fig 1B).
Among pts who reported reduced sexual activity in past 3 months (CD, US:69%, EU5:56%; UC, US:63%, EU5:53%), 38% US and 30% EU5 pts with CD, and 29% US and 18% EU5 pts with UC attributed it to CD/UC-related fatigue.
Discussion: Fatigue is a prevalent symptom of moderate-to-severe CD/UC which adversely affects pts’ work/school, social, physical, and sexual activities. This underscores the need to develop fatigue-related treatment strategies in CD and UC.

Figure: Figure 1. Impact of fatigue on health-related quality of life of patients with CD/UC (A) Patients who declined participation in work/school, social activities, and sports/physical exercise in the past three months due to CD/UC-related fatigue (B) Impact of CD/UC-related fatigue on work/school among patients who reported ever experiencing fatigue.
CD, Crohn's disease; UC, Ulcerative colitis; US, United States.
CD, Crohn's disease; UC, Ulcerative colitis; US, United States.
Note: The table for this abstract can be viewed in the ePoster Gallery section of the ACG 2024 ePoster Site or in The American Journal of Gastroenterology's abstract supplement issue, both of which will be available starting October 27, 2024.
Disclosures:
Remo Panaccione: Élan – Consultant. Abbivax – Consultant. Abbott – Consultant. AbbVie – Advisory Committee/Board Member, Consultant, Speaking Fees. Alimentiv – Advisory Committee/Board Member, Consultant. Amgen – Advisory Committee/Board Member, Consultant, Speaker Fees. Arena Pharmaceuticals – Advisory Committee/Board Member, Consultant, Speaker Fees. AstraZeneca – Advisory Committee/Board Member, Consultant. Biogen – Advisory Committee/Board Member, Consultant. Boehringer Ingelheim – Advisory Committee/Board Member, Consultant. Bristol Myers Squibb – Advisory Committee/Board Member, Consultant, Speaker Fees. Celgene – Advisory Committee/Board Member, Consultant, Speaker Fees. Celltrion – Consultant. Cosmos Pharmaceuticals – Consultant. Eisai – Consultant. Ferring – Advisory Committee/Board Member, Consultant, Speaker Fees. Fresenius Kabi – Advisory Committee/Board Member, Consultant, Speaker Fees. Galapagos – Consultant. Genentech – Advisory Committee/Board Member, Consultant. Gilead – Advisory Committee/Board Member, Consultant, Speaker Fees. GlaxoSmithKline – Advisory Committee/Board Member, Consultant. JAMP Bio – Advisory Committee/Board Member, Consultant. Janssen – Advisory Committee/Board Member, Consultant, Speaker Fees. Lilly – Advisory Committee/Board Member, Consultant, Speaker Fees. Merck – Advisory Committee/Board Member, Consultant, Speaker Fees. Mylan – Advisory Committee/Board Member, Consultant. Novartis – Advisory Committee/Board Member, Consultant. Oppilan Pharma – Advisory Committee/Board Member, Consultant. Organon – Advisory Committee/Board Member, Consultant, Speaker Fees. Pandion Pharma – Advisory Committee/Board Member, Consultant. Pendopharm – Consultant. Pfizer Inc – Advisory Committee/Board Member, Consultant, Speaker Fees. Progenity – Advisory Committee/Board Member, Consultant. Prometheus Biosciences – Consultant. Protagonist Therapeutics – Advisory Committee/Board Member, Consultant. Roche – Advisory Committee/Board Member, Consultant, Speaker Fees. Sandoz – Advisory Committee/Board Member, Consultant, Speaker Fees. Satisfai Health – Consultant. Shire – Advisory Committee/Board Member, Consultant, Speaker Fees. Sublimity Therapeutics – Advisory Committee/Board Member, Consultant. Takeda – Advisory Committee/Board Member, Consultant, Speaker Fees. Theravance – Consultant. Trellus – Consultant. UCB – Consultant. Ventyx – Advisory Committee/Board Member, Consultant. Viatris – Consultant.
Alison Potts Bleakman: Eli Lilly and Company – Employee, Stock Options.
Stefan Schreiber: AbbVie – Consultant, Personal fees, Speakers Bureau. Amgen – Personal fees. Arena Pharmaceuticals – Consultant, Personal fees, Speakers Bureau. Biogen – Consultant, Personal fees, Speakers Bureau. Bristol Myers Squibb – Consultant, Personal fees, Speakers Bureau. Celgene – Consultant, Personal fees, Speakers Bureau. Celltrion – Consultant, Personal fees, Speakers Bureau. Eli Lilly and Company – Personal fees. Falk – Consultant, Personal fees, Speakers Bureau. Ferring Pharmaceuticals – Personal fees. Fresenius – Consultant, Personal fees, Speakers Bureau. Galapagos – Personal fees. Gilead – Consultant, Personal fees. Hikma Pharmaceuticals – Advisory Committee/Board Member, Consultant. I-MAB – Consultant, Personal fees. Janssen – Consultant, Personal fees, Speakers Bureau. Morphic – Personal fees. MSD – Consultant, Personal fees, Speakers Bureau. Mylan – Consultant, Personal fees. Novartis – Personal fees. Pfizer Inc – Consultant, Personal fees, Speakers Bureau. Protagonist – Consultant, Personal fees. Provention Bio – Consultant, Personal fees. Roche – Personal fees. Sandoz/Hexal – Personal fees. Shire – Personal fees. Takeda – Consultant, Personal fees, Speakers Bureau. Theravance Biopharma – Consultant, Personal fees. Ventyx – Consultant, Personal fees.
Simon Travis: AbbVie – Grant/Research Support. Alimentiv – Consulting fees. Amgen – Consulting fees. Apexian – Consulting fees. Apollo – Consulting fees. Arcturis – Consulting fees. Arena – Consulting fees. AstraZeneca – Consulting fees. Biogen – Consulting fees. BMS – Consulting fees and Speaker fees. Buhlmann – Grant/Research Support, Consulting fees. Celgene – Grant/Research Support, Consulting fees. Celsius – Consulting fees. ChemoCentryx – Consulting fees. Clario – Consulting fees. Cosmo – Consulting fees. Dynavax – Consulting fees. ECCO – Grant/Research Support. Eli Lilly and Company – Grant/Research Support, Consulting fees and Speaker fees. Endpoint Health – Consulting fees. Enterome – Consulting fees. EQrX – Consulting fees. Equillium – Consulting fees. Ferring – Grant/Research Support, Speaker Fees. Galapagos – Grant/Research Support, Consulting fees. Genentech/Roche – Consulting fees. Gilead – Consulting fees. GSK – Grant/Research Support, Consulting fees. Helmsley Trust – Grant/Research Support. IOIBD – Grant/Research Support. Janssen – Grant/Research Support, Consulting fees and Speaker fees. Mestag – Consulting fees. Microbiotica – Consulting fees. Norman Collisson Foundation – Grant/Research Support. ONO – Consulting fees. Pfizer – Grant/Research Support, Consulting fees and Speaker fees. Protagonist – Consulting fees. Protagonist – Consulting fees. Satisfai Health – Consulting fees, Stock Options. Sensyne Health – Consulting fees. Sorriso – Consulting fees. Syndermix – Consulting fees. Takeda – Grant/Research Support, Consulting fees and Speaker fees. Theravance – Consulting fees. Topivert – Consulting fees. Tr1X Bio – Consulting fees. UCB Pharma – Consulting fees. UKIERI – Grant/Research Support. Vifor – Grant/Research Support, Consulting fees.
Marla Dubinsky: AbbVie – Consultant. Abivax – Consultant. Arena – Consultant. AstraZeneca – Consultant. Bristol Myers Squibb – Consultant. Celgene – Consultant. Eli Lilly – Consultant. Genentech – Consultant. Gilead – Consultant. Janssen – Consultant. Pfizer Inc – Consultant. Prometheus Labs – Consultant. Takeda – Consultant.
Toshifumi Hibi: AbbVie GK – Grant/Research Support. ActivAid – Grant/Research Support. Alfresa Pharma Corporation – Grant/Research Support, study group sponsorship. Bristol-Myers Squibb – Grant/Research Support. Celltrion – Advisory/consultancy fees. EA Pharma – Lecture fees. Eli Lilly and Company – Grant/Research Support, Advisory/consultancy fees. Ferring Pharmaceuticals – Grant/Research Support. Gilead Sciences – Grant/Research Support. Janssen – Grant/Research Support. JIMRO – study group sponsorship and lecture fees. JMDC Inc. – Grant/Research Support. Kyorin – study group sponsorship and lecture fees. Mitsubishi-Tanabe Pharma Corporation – Advisory/consultancy fees and lecture fees. MIYARISAN Pharmaceutical – study group sponsorship. Mochida Pharmaceutical – Grant/Research Support, study group sponsorship and lecture fees. Nippon Kayaku Co. – Grant/Research Support. Pfizer – Grant/Research Support, Lecture fees. Takeda Pharmaceutical – Grant/Research Support, Advisory/consultancy fees and lecture fees. Zeria Pharmaceutical – study group sponsorship and lecture fees.
Theresa Gibble: Eli Lilly and Company – Employee, Stock Options.
Tommaso Panni: Eli Lilly and Company – Employee. Eli Lilly and Company – Employee, Stock Options. Eli Lilly and Company – Stock Options.
Cem Kayhan: Eli Lilly and Company – Employee, Stock Options.
Eoin Flynn: Eli Lilly and Company – Employee, Stock Options.
Angelo Favia: Eli Lilly and Company – Employee, Stock Options.
Christian Atkinson: Adelphi Real World – Employee. Eli Lilly and Company (in connection with this study) – Consultant.
David Rubin: AbbVie – Consultant. AltruBio – Consultant. Apex – Consultant. Avalo Therapeutics – Consultant. Bausch Health – Consultant. Bristol Myers Squibb – Consultant. Buhlmann Diagnostics Corp – Consultant. Celgene – Consultant. ClostraBio – Consultant. Connect BioPharma – Consultant. Cornerstones Health – Board of Directors. Crohn's & Colitis Foundation – Board of Trustees. Douglas Therapeutics – Consultant. Eli Lilly – Consultant. InDex Pharmaceuticals – Consultant. Intouch Group – Consultant. Iterative Health – Consultant. Janssen Pharmaceuticals – Consultant. Odyssey Thera – Consultant. Pfizer – Consultant. Prometheus Biosciences – Consultant. Samsung Neurologica – Consultant. Takeda – Consultant, Grant/Research Support.
Remo Panaccione, MD1, Alison Potts Bleakman, PhD2, Stefan Schreiber, MD3, Simon Travis, MBBS, MRCP, PhD4, Marla C. Dubinsky, MD5, Toshifumi Hibi, MD, PhD6, Theresa Gibble, PhD2, Tommaso Panni, PhD2, Cem Kayhan, MD2, Eoin Flynn, PhD2, Angelo Favia, PhD2, Christian Atkinson, MRes7, David T.. Rubin, MD, FACG8. P4380 - Burden and Impact of Fatigue on Patients with Crohn’s Disease and Ulcerative Colitis in the United States and Europe: Results from the Communicating Needs and Features of IBD Experiences (CONFIDE) Survey, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
