Tuesday Poster Session
Category: IBD
P4381 - Safety of Long-Term Ozanimod Treatment Up to 5 Years in Patients With Moderately to Severely Active Ulcerative Colitis: An Interim Analysis of the True North Open-Label Extension
Tuesday, October 29, 2024
10:30 AM - 4:00 PM ET
Location: Exhibit Hall E

Bincy Abraham, MD, MS, FACG
Houston Methodist-Weill Cornell
Houston, TX
Presenting Author(s)
Bincy P. Abraham, MD, MS, FACG1, Randy Longman, MD, PhD2, Konstantinos Katsanos, MD, MBA, PhD, FEBGH3, Scott D. Lee, MD4, Taku Kobayashi, MD5, Dominik Bettenworth, MD6, Louis Korman, MD7, Dimpy Mehra, PharmD8, Norma Ruiz Santiago, MD8, AnnKatrin Petersen, MD8, Manik Desai, PhD8, Hsiuanlin Wu, MS8, Dong Wang, PhD9, Mark T. Osterman, MD8, Anjali Jain, PhD8, Joana Torres, MD, PhD10
1Houston Methodist-Weill Cornell, Houston, TX; 2Weill Cornell Medicine, New York, NY; 3University of Ioannina, Ioannina, Ioannina, Greece; 4Digestive Health Center, University of Washington Medical Center, Seattle, WA; 5Kitasato University Kitasato Institute Hospital, Minato City, Tokyo, Japan; 6University of Münster, CED Schwerpunktpraxis, Münster, Hamburg, Germany; 7Capital Digestive Care, Washington, DC; 8Bristol Myers Squibb, Princeton, NJ; 9Bristol Myers Squibb K. K., Chiyoda City, Tokyo, Japan; 10Hospital da Luz, Hospital Beatriz Angelo, Faculdade de Medicina, Universidade de Lisboa, Lisbon, Lisboa, Portugal
Introduction: The 52-week, phase 3 True North (TN) study (NCT02435992) demonstrated the efficacy and safety of ozanimod (OZA), a highly selective sphingosine 1-phosphate receptor 1 and 5 modulator, in patients (pts) with moderately to severely active ulcerative colitis (UC). A prior analysis reported the long-term safety of OZA up to ~4 y from TN baseline through Week (W) 142 of the ongoing TN open-label extension (OLE; NCT02531126).
Methods: This analysis assessed the cumulative long-term safety of OZA with an additional year of exposure (pt disposition available until OLE W190 [up to ~5 y of OZA]). All pts who entered the OLE from TN (ie, clinical nonresponders at W10 and those who lost response during maintenance or completed maintenance at W52) were included in this analysis. Treatment-emergent adverse events (TEAEs) were monitored from the first dose of OZA in TN or the OLE through data cutoff; exposure-adjusted incidence rates (EAIRs) per 100 patient-years (PY) are presented. Lab abnormalities, including absolute lymphocyte count (ALC) reductions, were assessed during the OLE through data cutoff.
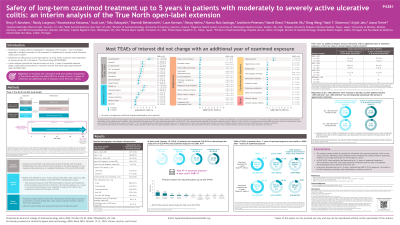
Results: A total of 823 pts entered the TN OLE. At data cutoff, 43.0% of pts had completed OLE W190 and total OZA exposure was 2681 PY. Overall, the cumulative safety assessments did not meaningfully change with an additional year of OZA exposure (Table). EAIRs of TEAEs, serious TEAEs, and TEAEs leading to treatment discontinuation remained consistent, and there were no new cases of malignancy, bradycardia, third-degree atrioventricular (AV) block, myocardial ischemia, ischemic stroke, pulmonary embolism, deep vein thrombosis, or macular edema with an additional year of OZA exposure. Three pts newly developed infections; however, only 1 pt had a serious infection (keratouveitis), which was not related to treatment. Two additional pts experienced mild hypertension. Reductions in ALC < 500 cells/µl were common in the OLE (57.2%), but few pts had ALC < 200 cells/µl (6.6%). Occurrence of ALC < 200 cells/µl was not temporally associated with serious or opportunistic infections. Most alanine aminotransferase and aspartate aminotransferase elevations were transient and resolved without treatment interruption, and no serious hepatic events occurred.
Discussion: The ongoing safety profile of OZA is consistent with previous analyses, with no new safety concerns identified. Long-term OZA use representing 2681 PY of exposure continues to be well tolerated in pts with moderately to severely active UC.
Note: The table for this abstract can be viewed in the ePoster Gallery section of the ACG 2024 ePoster Site or in The American Journal of Gastroenterology's abstract supplement issue, both of which will be available starting October 27, 2024.
Disclosures:
Bincy P. Abraham, MD, MS, FACG1, Randy Longman, MD, PhD2, Konstantinos Katsanos, MD, MBA, PhD, FEBGH3, Scott D. Lee, MD4, Taku Kobayashi, MD5, Dominik Bettenworth, MD6, Louis Korman, MD7, Dimpy Mehra, PharmD8, Norma Ruiz Santiago, MD8, AnnKatrin Petersen, MD8, Manik Desai, PhD8, Hsiuanlin Wu, MS8, Dong Wang, PhD9, Mark T. Osterman, MD8, Anjali Jain, PhD8, Joana Torres, MD, PhD10. P4381 - Safety of Long-Term Ozanimod Treatment Up to 5 Years in Patients With Moderately to Severely Active Ulcerative Colitis: An Interim Analysis of the True North Open-Label Extension, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
1Houston Methodist-Weill Cornell, Houston, TX; 2Weill Cornell Medicine, New York, NY; 3University of Ioannina, Ioannina, Ioannina, Greece; 4Digestive Health Center, University of Washington Medical Center, Seattle, WA; 5Kitasato University Kitasato Institute Hospital, Minato City, Tokyo, Japan; 6University of Münster, CED Schwerpunktpraxis, Münster, Hamburg, Germany; 7Capital Digestive Care, Washington, DC; 8Bristol Myers Squibb, Princeton, NJ; 9Bristol Myers Squibb K. K., Chiyoda City, Tokyo, Japan; 10Hospital da Luz, Hospital Beatriz Angelo, Faculdade de Medicina, Universidade de Lisboa, Lisbon, Lisboa, Portugal
Introduction: The 52-week, phase 3 True North (TN) study (NCT02435992) demonstrated the efficacy and safety of ozanimod (OZA), a highly selective sphingosine 1-phosphate receptor 1 and 5 modulator, in patients (pts) with moderately to severely active ulcerative colitis (UC). A prior analysis reported the long-term safety of OZA up to ~4 y from TN baseline through Week (W) 142 of the ongoing TN open-label extension (OLE; NCT02531126).
Methods: This analysis assessed the cumulative long-term safety of OZA with an additional year of exposure (pt disposition available until OLE W190 [up to ~5 y of OZA]). All pts who entered the OLE from TN (ie, clinical nonresponders at W10 and those who lost response during maintenance or completed maintenance at W52) were included in this analysis. Treatment-emergent adverse events (TEAEs) were monitored from the first dose of OZA in TN or the OLE through data cutoff; exposure-adjusted incidence rates (EAIRs) per 100 patient-years (PY) are presented. Lab abnormalities, including absolute lymphocyte count (ALC) reductions, were assessed during the OLE through data cutoff.
Results: A total of 823 pts entered the TN OLE. At data cutoff, 43.0% of pts had completed OLE W190 and total OZA exposure was 2681 PY. Overall, the cumulative safety assessments did not meaningfully change with an additional year of OZA exposure (Table). EAIRs of TEAEs, serious TEAEs, and TEAEs leading to treatment discontinuation remained consistent, and there were no new cases of malignancy, bradycardia, third-degree atrioventricular (AV) block, myocardial ischemia, ischemic stroke, pulmonary embolism, deep vein thrombosis, or macular edema with an additional year of OZA exposure. Three pts newly developed infections; however, only 1 pt had a serious infection (keratouveitis), which was not related to treatment. Two additional pts experienced mild hypertension. Reductions in ALC < 500 cells/µl were common in the OLE (57.2%), but few pts had ALC < 200 cells/µl (6.6%). Occurrence of ALC < 200 cells/µl was not temporally associated with serious or opportunistic infections. Most alanine aminotransferase and aspartate aminotransferase elevations were transient and resolved without treatment interruption, and no serious hepatic events occurred.
Discussion: The ongoing safety profile of OZA is consistent with previous analyses, with no new safety concerns identified. Long-term OZA use representing 2681 PY of exposure continues to be well tolerated in pts with moderately to severely active UC.
Note: The table for this abstract can be viewed in the ePoster Gallery section of the ACG 2024 ePoster Site or in The American Journal of Gastroenterology's abstract supplement issue, both of which will be available starting October 27, 2024.
Disclosures:
Bincy P. Abraham: AbbVie – Consultant, Speakers Bureau. Bristol Myers Squibb – Consultant, Speakers Bureau. Celltrion – Consultant. Eli Lilly – Consultant, Speakers Bureau. Fresenius Kabi – Consultant. Janssen – Consultant, Speakers Bureau. Medtronic – Consultant. Pfizer – Consultant, Speakers Bureau. Prometheus – Consultant. Samsung Bioepis – Consultant. Takeda – Consultant, Speakers Bureau.
Randy Longman: Bristol Myers Squibb – Consultant. Pfizer – Consultant.
Konstantinos Katsanos: Gore – lecture honoraria.
Scott Lee: AbbVie – Consultant. Bristol Myers Squibb – Consultant. Eli Lilly – Consultant. Janssen – Consultant.
Taku Kobayashi: AbbVie – Advisory Committee/Board Member, Consultant, Grant/Research Support, Speakers Bureau. Alfresa Pharma – Advisory Committee/Board Member, Consultant, Grant/Research Support, Speakers Bureau. Alimentiv – Advisory Committee/Board Member, Consultant, Speakers Bureau. Astellas – Advisory Committee/Board Member, Consultant, Speakers Bureau. Bristol Myers Squibb – Advisory Committee/Board Member, Consultant, Speakers Bureau. Celltrion – Advisory Committee/Board Member, Consultant, Speakers Bureau. Covidien – Advisory Committee/Board Member, Consultant, Speakers Bureau. EA Pharma – Advisory Committee/Board Member, Consultant, Grant/Research Support, Speakers Bureau. Eli Lilly – Advisory Committee/Board Member, Consultant, Speakers Bureau. Ferring – Advisory Committee/Board Member, Consultant, Speakers Bureau. Galapagos – Advisory Committee/Board Member, Consultant, Speakers Bureau. Gilead Sciences – Advisory Committee/Board Member, Consultant, Grant/Research Support, Speakers Bureau. Janssen – Advisory Committee/Board Member, Consultant, Speakers Bureau. JIMRO – Advisory Committee/Board Member, Consultant, Speakers Bureau. JMDC – Advisory Committee/Board Member, Consultant, Speakers Bureau. Kissei Pharmaceutical – Advisory Committee/Board Member, Consultant, Speakers Bureau. Kyorin Pharmaceutical – Advisory Committee/Board Member, Consultant, Grant/Research Support, Speakers Bureau. Mitsubishi Tanabe Pharma – Advisory Committee/Board Member, Consultant, Speakers Bureau. Mochida Pharmaceutical – Advisory Committee/Board Member, Consultant, Grant/Research Support, Speakers Bureau. Nippon Kayaku – Advisory Committee/Board Member, Consultant, Grant/Research Support, Speakers Bureau. Otsuka Holdings – Grant/Research Support. Pfizer – Advisory Committee/Board Member, Consultant, Grant/Research Support, Speakers Bureau. Sekisui Medical – Grant/Research Support. Takeda – Advisory Committee/Board Member, Consultant, Grant/Research Support, Speakers Bureau. ThermoFisher Scientific – Advisory Committee/Board Member, Consultant, Grant/Research Support, Speakers Bureau. Zeria Pharmaceutical – Advisory Committee/Board Member, Consultant, Grant/Research Support, Speakers Bureau.
Dominik Bettenworth: AbbVie – Advisory Committee/Board Member, Consultant. Amgen – Advisory Committee/Board Member, Consultant. Arena – Advisory Committee/Board Member, Consultant. Atheneum – Advisory Committee/Board Member, Consultant. BNG Service GmbH – Advisory Committee/Board Member, Consultant. Bristol Myers Squibb – Advisory Committee/Board Member, Consultant. CED Service GmbH – Advisory Committee/Board Member, Consultant. Celltrion – Advisory Committee/Board Member, Consultant. DGVS – Advisory Committee/Board Member, Consultant. Diaplan – Advisory Committee/Board Member, Consultant. Doctorflix – Advisory Committee/Board Member, Consultant. Eli Lilly – Advisory Committee/Board Member, Consultant. Else Kröner-Fresenius-Stiftung – Advisory Committee/Board Member, Consultant. Falk Foundation – Advisory Committee/Board Member, Consultant. Ferring – Advisory Committee/Board Member, Consultant. Galapagos – Advisory Committee/Board Member, Consultant. Gastroenterology Today – Advisory Committee/Board Member, Consultant. GlaxoSmithKline – Advisory Committee/Board Member, Consultant. Guidepoint – Advisory Committee/Board Member, Consultant. Impulze – Advisory Committee/Board Member, Consultant. Janssen-Cilag – Advisory Committee/Board Member, Consultant. Medical Tribune – Advisory Committee/Board Member, Consultant. MedTrix – Advisory Committee/Board Member, Consultant. MSD – Advisory Committee/Board Member, Consultant. Mylan – Advisory Committee/Board Member, Consultant. Onkowissen – Advisory Committee/Board Member, Consultant. Pfizer – Advisory Committee/Board Member, Consultant. Pharmacosmos – Advisory Committee/Board Member, Consultant. Roche – Advisory Committee/Board Member, Consultant. Sandoz – Advisory Committee/Board Member, Consultant. Streamed Up – Advisory Committee/Board Member, Consultant. Takeda – Advisory Committee/Board Member, Consultant. Tetrameros – Advisory Committee/Board Member, Consultant. Thieme – Advisory Committee/Board Member, Consultant. Tillotts – Advisory Committee/Board Member, Consultant. UCB – Advisory Committee/Board Member, Consultant. Viatris – Advisory Committee/Board Member, Consultant. Vifor Pharma – Advisory Committee/Board Member, Consultant.
Louis Korman indicated no relevant financial relationships.
Dimpy Mehra: Bristol Myers Squibb – Employee.
Norma Ruiz Santiago: Bristol Myers Squibb – Employee.
AnnKatrin Petersen: Bristol Myers Squibb – Employee.
Manik Desai: Bristol Myers Squibb – Employee.
Hsiuanlin Wu: Bristol Myers Squibb – Employee.
Dong Wang: Bristol Myers Squibb – Employee.
Mark T. Osterman: Bristol Myers Squibb – Employee.
Anjali Jain: Bristol Myers Squibb – Employee.
Joana Torres: AbbVie – Advisory Committee/Board Member, Grant/Research Support, Speakers Bureau. Arena – Advisory Committee/Board Member, Speaker Fees. Bristol Myers Squibb – Advisory Committee/Board Member, Speakers Bureau. Janssen – Advisory Committee/Board Member, Grant/Research Support, Speakers Bureau. Pfizer – Advisory Committee/Board Member, Speakers Bureau. Sandoz – Advisory Committee/Board Member, Speakers Bureau.
Bincy P. Abraham, MD, MS, FACG1, Randy Longman, MD, PhD2, Konstantinos Katsanos, MD, MBA, PhD, FEBGH3, Scott D. Lee, MD4, Taku Kobayashi, MD5, Dominik Bettenworth, MD6, Louis Korman, MD7, Dimpy Mehra, PharmD8, Norma Ruiz Santiago, MD8, AnnKatrin Petersen, MD8, Manik Desai, PhD8, Hsiuanlin Wu, MS8, Dong Wang, PhD9, Mark T. Osterman, MD8, Anjali Jain, PhD8, Joana Torres, MD, PhD10. P4381 - Safety of Long-Term Ozanimod Treatment Up to 5 Years in Patients With Moderately to Severely Active Ulcerative Colitis: An Interim Analysis of the True North Open-Label Extension, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
