Tuesday Poster Session
Category: IBD
P4395 - Dose Intensification of Risankizumab for Crohn’s Disease: Access and Outcomes
Tuesday, October 29, 2024
10:30 AM - 4:00 PM ET
Location: Exhibit Hall E
Has Audio

Rahul Dalal, MD, MPH
Brigham and Women's Hospital
Boston, MA
Presenting Author(s)
Rahul Dalal, MD, MPH1, Heidy Cabral, BS, MS1, Alexander Carlin, BS1, Jessica R. Allegretti, MD, MPH, FACG2
1Brigham and Women's Hospital, Boston, MA; 2Brigham and Women’s Hospital, Harvard Medical School, Boston, MA
Introduction: Prior research has demonstrated the effectiveness of empiric dose intensification of ustekinumab, an IL-12/23 antagonist, to recapture clinical response for Crohn’s disease. It is unknown if dose intensification of risankizumab, an IL-23 antagonist, is similarly effective. We described access to risankizumab dose intensification for Crohn’s disease and subsequent clinical outcomes at 8-16 weeks.
Methods: In this retrospective cohort study, adults with Crohn’s disease underwent empiric dose intensification of maintenance subcutaneous risankizumab from 360 mg every 8 weeks to every 6 (q6w) or every 4 weeks (q4w) at a large academic health system. We described access to intensified dosing regimens in terms of insurance approval and reported clinical outcomes. Clinical outcomes included change in the Harvey Bradshaw Index (HBI) from pre-dose intensification to first clinical assessment at 8-16 weeks after dose intensification, clinical response (i.e. improvement in HBI >3 points or HBI < 5) at 8-16 weeks, steroid-free clinical remission (SFCR; i.e. HBI < 5 and no use of oral steroids) at 8-16 weeks, endoscopic improvement (when data were available), and adverse events (AEs).
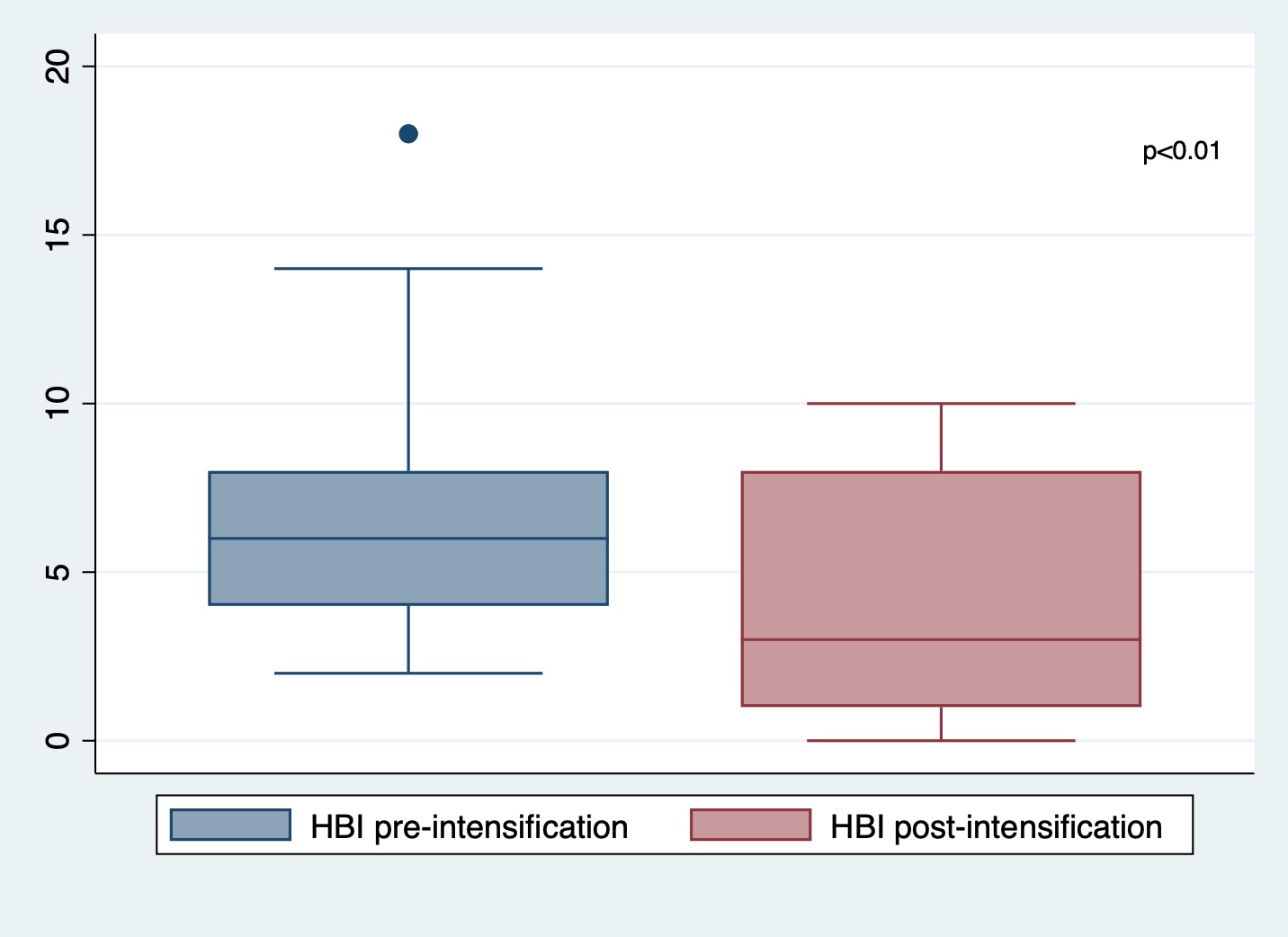
Results: Between 5/1/2023-5/31/2024, 30 patients on maintenance risankizumab were prescribed intensified dosing regimens for loss of response or incomplete response to therapy. 14 (47%) prescriptions were initially approved by insurance and 16 (53%) were denied and then appealed. Ten (63%) appeals were ultimately successful after a median of 23 days (IQR 15-43 days). 17 patients had clinical data available both pre- and post-dose intensification (n=8 for q4w, n=9 for q6w) at 8-16 weeks. Baseline characteristics are presented in Table 1. There was a significant improvement in mean HBI scores from 6.9 to 3.8 pre vs post-dose intensification (p< 0.01 by paired t-test; Figure 1). 12 (71%) achieved clinical response and 9 (53%) achieved SFCR. 4/6 (67%) had endoscopic improvement, 1/6 (17%) had no change, and 1/6 (17%) had endoscopic worsening after dose intensification per endoscopist assessment. No AEs were reported during follow-up and one patient (6%) discontinued therapy due to non-response.
Discussion: Dose intensification of risankizumab to q6w or q4w appears to be effective in achieving clinical response for the majority of patients with suboptimal response to standard dosing, however access is currently limited. Larger cohort studies are needed to compare optimized dosing regimens and assess long-term outcomes.
Note: The table for this abstract can be viewed in the ePoster Gallery section of the ACG 2024 ePoster Site or in The American Journal of Gastroenterology's abstract supplement issue, both of which will be available starting October 27, 2024.
Disclosures:
Rahul Dalal, MD, MPH1, Heidy Cabral, BS, MS1, Alexander Carlin, BS1, Jessica R. Allegretti, MD, MPH, FACG2. P4395 - Dose Intensification of Risankizumab for Crohn’s Disease: Access and Outcomes, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
1Brigham and Women's Hospital, Boston, MA; 2Brigham and Women’s Hospital, Harvard Medical School, Boston, MA
Introduction: Prior research has demonstrated the effectiveness of empiric dose intensification of ustekinumab, an IL-12/23 antagonist, to recapture clinical response for Crohn’s disease. It is unknown if dose intensification of risankizumab, an IL-23 antagonist, is similarly effective. We described access to risankizumab dose intensification for Crohn’s disease and subsequent clinical outcomes at 8-16 weeks.
Methods: In this retrospective cohort study, adults with Crohn’s disease underwent empiric dose intensification of maintenance subcutaneous risankizumab from 360 mg every 8 weeks to every 6 (q6w) or every 4 weeks (q4w) at a large academic health system. We described access to intensified dosing regimens in terms of insurance approval and reported clinical outcomes. Clinical outcomes included change in the Harvey Bradshaw Index (HBI) from pre-dose intensification to first clinical assessment at 8-16 weeks after dose intensification, clinical response (i.e. improvement in HBI >3 points or HBI < 5) at 8-16 weeks, steroid-free clinical remission (SFCR; i.e. HBI < 5 and no use of oral steroids) at 8-16 weeks, endoscopic improvement (when data were available), and adverse events (AEs).
Results: Between 5/1/2023-5/31/2024, 30 patients on maintenance risankizumab were prescribed intensified dosing regimens for loss of response or incomplete response to therapy. 14 (47%) prescriptions were initially approved by insurance and 16 (53%) were denied and then appealed. Ten (63%) appeals were ultimately successful after a median of 23 days (IQR 15-43 days). 17 patients had clinical data available both pre- and post-dose intensification (n=8 for q4w, n=9 for q6w) at 8-16 weeks. Baseline characteristics are presented in Table 1. There was a significant improvement in mean HBI scores from 6.9 to 3.8 pre vs post-dose intensification (p< 0.01 by paired t-test; Figure 1). 12 (71%) achieved clinical response and 9 (53%) achieved SFCR. 4/6 (67%) had endoscopic improvement, 1/6 (17%) had no change, and 1/6 (17%) had endoscopic worsening after dose intensification per endoscopist assessment. No AEs were reported during follow-up and one patient (6%) discontinued therapy due to non-response.
Discussion: Dose intensification of risankizumab to q6w or q4w appears to be effective in achieving clinical response for the majority of patients with suboptimal response to standard dosing, however access is currently limited. Larger cohort studies are needed to compare optimized dosing regimens and assess long-term outcomes.

Figure: Figure 1. HBI score distributions pre vs post-dose intensification.
Abbreviations: HBI = Harvey Bradshaw Index
Abbreviations: HBI = Harvey Bradshaw Index
Note: The table for this abstract can be viewed in the ePoster Gallery section of the ACG 2024 ePoster Site or in The American Journal of Gastroenterology's abstract supplement issue, both of which will be available starting October 27, 2024.
Disclosures:
Rahul Dalal: Centaur Labs – Consultant. Janssen – Consultant, Grant/Research Support. Pfizer – Grant/Research Support. Takeda – Consultant.
Heidy Cabral indicated no relevant financial relationships.
Alexander Carlin indicated no relevant financial relationships.
Jessica R. Allegretti: Abbvie – Consultant, Speakers Bureau. Artugen – Consultant. Bristol Myers Squibb – Consultant, Speakers Bureau. Ferring – Consultant. Finch Therapeutics – Consultant. Janssen – Consultant. Merck – Grant/Research Support. Pfizer – Consultant. Seres – Consultant.
Rahul Dalal, MD, MPH1, Heidy Cabral, BS, MS1, Alexander Carlin, BS1, Jessica R. Allegretti, MD, MPH, FACG2. P4395 - Dose Intensification of Risankizumab for Crohn’s Disease: Access and Outcomes, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.

