Tuesday Poster Session
Category: IBD
P4399 - Efficacy and Safety of GLP-1 Agonists on Metabolic Parameters in Non-Diabetic Patients With Inflammatory Bowel Disease
Tuesday, October 29, 2024
10:30 AM - 4:00 PM ET
Location: Exhibit Hall E
Has Audio

Jeremy A. Klein, MD
University of Chicago Medical Center
Chicago, IL
Presenting Author(s)
Jeremy A. Klein, MD1, Joelle St-Pierre, MD, PhD2, Natalie K. Choi, BA2, Evan N. Fear, BA2, David T.. Rubin, MD, FACG2
1University of Chicago Medical Center, Chicago, IL; 2University of Chicago Medicine, Inflammatory Bowel Disease Center, Chicago, IL
Introduction: Although glucagon-like peptide-1 (GLP-1) agonists have shown promise in weight reduction and metabolic control in non-diabetic populations, GLP-1 efficacy/safety in patients with IBD is underexplored. This study aims to evaluate the impact of GLP-1 therapy on weight loss and metabolic parameters in non-diabetic IBD patients.
Methods: We conducted a single-center observational cohort study that included adult patients with IBD who started GLP-1 (semaglutide or tirzepatide) for weight loss from January 2021 to April 2024. Patients with diabetes were excluded. The primary outcome was a change in BMI and total body weight (TBW). Secondary outcomes included tolerability, safety, and changes in metabolic risk factors. Continuous variables are presented as medians and interquartile (IQR) ranges. GraphPad Prism was used for statistical analysis including paired and unpaired t-tests.
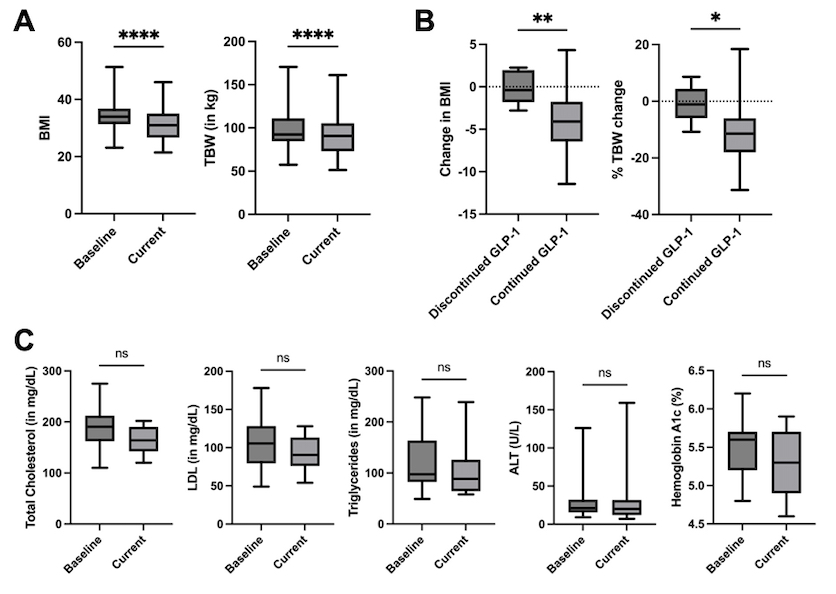
Results: The study included 36 patients with IBD (64% female (64%), median age 45.5 years (IQR 41-51.5 years), 31% ulcerative colitis (UC), 67% had Crohn's disease (CD)). Most patients (86%) had prior repeated systemic steroid exposure, and 86% were receiving advanced therapy. Most patients (83%) were in remission from IBD throughout the study. Of the six patients that changed IBD therapy, four had signs of inflammation prior to GLP-1 initiation. Co-morbidities included dyslipidemia (39%), metabolic-associated steatotic liver disease (31%), and medicated hypertension (25%). In all GLP-1-exposed patients, BMI significantly decreased from 34.0 (IQR 32.5-38.2) to 31.0 (IQR 26.5-36.1) (p< 0.0001). Similarly, TBW significantly decreased by a median of 8.15 kg (IQR 15.9-2.2 kg; p< 0.0001) (Figure 1A). Although a decrease in total cholesterol and glycosylated hemoglobin was seen, it was not statistically significant (p=0.0634 and p=0.0536, respectively) (Figure 1C). The most common side effects were nausea (31%) and constipation (25%). Eight patients (22%) discontinued GLP-1 therapy due to insurance coverage (4/8 patients) and GI intolerance (3/8). Patients who discontinued GLP-1 showed a median change in BMI of -0.4 (IQR -1.7-1.7; p=0.0042) (Figure 1B, left panel).
Discussion: Our study shows that GLP-1-based therapy can effectively reduce BMI and weight in non-diabetic IBD patients. In addition, GLP-1 is well tolerated and not associated with worsening of IBD activity. Further prospective studies with larger sample sizes are needed to explore the long-term implications of GLP-1 agonists in the IBD population.
Disclosures:
Jeremy A. Klein, MD1, Joelle St-Pierre, MD, PhD2, Natalie K. Choi, BA2, Evan N. Fear, BA2, David T.. Rubin, MD, FACG2. P4399 - Efficacy and Safety of GLP-1 Agonists on Metabolic Parameters in Non-Diabetic Patients With Inflammatory Bowel Disease, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
1University of Chicago Medical Center, Chicago, IL; 2University of Chicago Medicine, Inflammatory Bowel Disease Center, Chicago, IL
Introduction: Although glucagon-like peptide-1 (GLP-1) agonists have shown promise in weight reduction and metabolic control in non-diabetic populations, GLP-1 efficacy/safety in patients with IBD is underexplored. This study aims to evaluate the impact of GLP-1 therapy on weight loss and metabolic parameters in non-diabetic IBD patients.
Methods: We conducted a single-center observational cohort study that included adult patients with IBD who started GLP-1 (semaglutide or tirzepatide) for weight loss from January 2021 to April 2024. Patients with diabetes were excluded. The primary outcome was a change in BMI and total body weight (TBW). Secondary outcomes included tolerability, safety, and changes in metabolic risk factors. Continuous variables are presented as medians and interquartile (IQR) ranges. GraphPad Prism was used for statistical analysis including paired and unpaired t-tests.
Results: The study included 36 patients with IBD (64% female (64%), median age 45.5 years (IQR 41-51.5 years), 31% ulcerative colitis (UC), 67% had Crohn's disease (CD)). Most patients (86%) had prior repeated systemic steroid exposure, and 86% were receiving advanced therapy. Most patients (83%) were in remission from IBD throughout the study. Of the six patients that changed IBD therapy, four had signs of inflammation prior to GLP-1 initiation. Co-morbidities included dyslipidemia (39%), metabolic-associated steatotic liver disease (31%), and medicated hypertension (25%). In all GLP-1-exposed patients, BMI significantly decreased from 34.0 (IQR 32.5-38.2) to 31.0 (IQR 26.5-36.1) (p< 0.0001). Similarly, TBW significantly decreased by a median of 8.15 kg (IQR 15.9-2.2 kg; p< 0.0001) (Figure 1A). Although a decrease in total cholesterol and glycosylated hemoglobin was seen, it was not statistically significant (p=0.0634 and p=0.0536, respectively) (Figure 1C). The most common side effects were nausea (31%) and constipation (25%). Eight patients (22%) discontinued GLP-1 therapy due to insurance coverage (4/8 patients) and GI intolerance (3/8). Patients who discontinued GLP-1 showed a median change in BMI of -0.4 (IQR -1.7-1.7; p=0.0042) (Figure 1B, left panel).
Discussion: Our study shows that GLP-1-based therapy can effectively reduce BMI and weight in non-diabetic IBD patients. In addition, GLP-1 is well tolerated and not associated with worsening of IBD activity. Further prospective studies with larger sample sizes are needed to explore the long-term implications of GLP-1 agonists in the IBD population.

Figure: Figure 1. Changes in metabolic parameters with GLP-1-based therapies. (A) BMI and TBW at baseline and after exposure to GLP-1-based therapy in all patients (p<0.0001 for BMI and TBW). (B) Changes in BMI and %TBW (%TBW) in patients who discontinued GLP-1-based therapy compared to those who continued therapy (p=0.0042 for BMI and p=0.0115 for %TBW). (C) Changes in total cholesterol (p=0.06), LDL (p=0.19), triglycerides (p=0.46), ALT (p=0.86), hemoglobin A1c (p=0.05) and CRP (p=0.21) levels at baseline and after exposure to GLP-1-based therapy in all patients.
Disclosures:
Jeremy Klein indicated no relevant financial relationships.
Joelle St-Pierre indicated no relevant financial relationships.
Natalie Choi indicated no relevant financial relationships.
Evan Fear indicated no relevant financial relationships.
David Rubin: AbbVie – Consultant. AltruBio – Consultant. Apex – Consultant. Avalo Therapeutics – Consultant. Bausch Health – Consultant. Bristol Myers Squibb – Consultant. Buhlmann Diagnostics Corp – Consultant. Celgene – Consultant. ClostraBio – Consultant. Connect BioPharma – Consultant. Cornerstones Health – Board of Directors. Crohn's & Colitis Foundation – Board of Trustees. Douglas Therapeutics – Consultant. Eli Lilly – Consultant. InDex Pharmaceuticals – Consultant. Intouch Group – Consultant. Iterative Health – Consultant. Janssen Pharmaceuticals – Consultant. Odyssey Thera – Consultant. Pfizer – Consultant. Prometheus Biosciences – Consultant. Samsung Neurologica – Consultant. Takeda – Consultant, Grant/Research Support.
Jeremy A. Klein, MD1, Joelle St-Pierre, MD, PhD2, Natalie K. Choi, BA2, Evan N. Fear, BA2, David T.. Rubin, MD, FACG2. P4399 - Efficacy and Safety of GLP-1 Agonists on Metabolic Parameters in Non-Diabetic Patients With Inflammatory Bowel Disease, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
