Tuesday Poster Session
Category: IBD
P4400 - Elevation of Liver Enzymes In Patients With Inflammatory Bowel Disease Treated With Upadacitinib Is Associated With Metabolic Risk Factors
Tuesday, October 29, 2024
10:30 AM - 4:00 PM ET
Location: Exhibit Hall E

Has Audio

Jeremy A. Klein, MD
University of Chicago Medical Center
Chicago, IL
Presenting Author(s)
Jeremy A. Klein, MD1, Joelle St-Pierre, MD, PhD2, Natalie K. Choi, BA2, Nicole Garcia, BA2, Emma A. Picker, BA2, David T.. Rubin, MD, FACG2
1University of Chicago Medical Center, Chicago, IL; 2University of Chicago Medicine, Inflammatory Bowel Disease Center, Chicago, IL
Introduction: Upadacitinib (UPA) is an oral Janus kinase inhibitor (JAKi) used to treat inflammatory bowel disease (IBD). Prior phase 3 clinical trials of UPA in IBD identified 2.5% to 7.4% of patients with liver enzyme elevations (defined as ALT 3X the upper limit of normal [ULN]). The frequency of mild liver enzyme elevations is not reported. We assessed the incidence of elevated liver enzymes in a real-world population of patients with IBD treated with UPA and explore patient- and disease-related risk factors for an elevated ALT.
Methods: This retrospective cohort study included patients with IBD at the University of Chicago who were treated for 1 year with UPA. Statistical difference between ALT at different time points compared to baseline (pre-UPA initiation) was done with Wilcoxon matched-pairs signed rank test. Spearman’s correlation was used to examine the relationship between liver enzyme elevation (defined as ALT >35 U/L), inflammatory markers (C-reactive protein [CRP], fecal calprotectin [FCP]) and metabolic risk factors (hypertension, dyslipidemia, metabolic dysfunction-associated steatotic liver disease [MASLD], and elevated BMI).
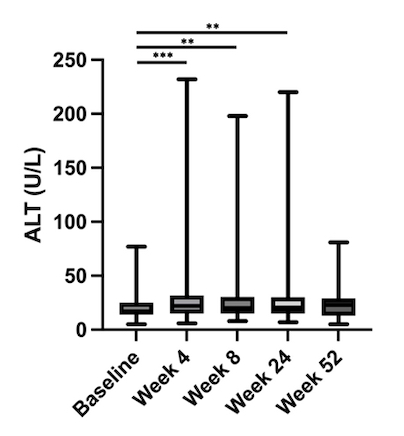
Results: 160 patients (50% female, median age 37) were included. Of these, 58.1% had UC, 35% had CD, 3.1% IBD unclassified, and 4.4% pouchitis with CD-like features. Elevated ALT in 15/151 (9.9%) at baseline and 32/160 patients (20%) within the first year after UPA initiation, with 3 patients (1.9%) having ALT > 3x ULN. Compared to baseline, ALT was significantly elevated at weeks 4 (p=0.0003), 8 (p=0.002) and 24 (p=0.002), but not week 52 (p=0.234) (Figure 1). Significant correlations were found between ALT elevation and BMI over 25 kg/m2 (r=0.256, p=0.001), HTN (r=0.209, p=0.008), DLD (r=0.315, p< 0.0001), and MASLD (r=0.258, p=0.001), and male gender (0.156, p=0.049). Cigarette smoking was not associated with ALT elevation (Table I). Correlation between ALT, CRP, and FCP at different time points was not associated with changes in ALT levels other than CRP at week 8 and FCP at month 6. No patients developed liver failure or had features of Hy’s law.
Discussion: Patients with IBD experienced mild elevations in ALT after UPA initiation, particularly among those with additional metabolic risk factors. These findings emphasize the importance of careful monitoring of these patients to mitigate liver-related adverse effects. Future prospective studies should further delineate the mechanisms and long-term implications of liver enzyme elevations in this population.
Note: The table for this abstract can be viewed in the ePoster Gallery section of the ACG 2024 ePoster Site or in The American Journal of Gastroenterology's abstract supplement issue, both of which will be available starting October 27, 2024.
Disclosures:
Jeremy A. Klein, MD1, Joelle St-Pierre, MD, PhD2, Natalie K. Choi, BA2, Nicole Garcia, BA2, Emma A. Picker, BA2, David T.. Rubin, MD, FACG2. P4400 - Elevation of Liver Enzymes In Patients With Inflammatory Bowel Disease Treated With Upadacitinib Is Associated With Metabolic Risk Factors, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
1University of Chicago Medical Center, Chicago, IL; 2University of Chicago Medicine, Inflammatory Bowel Disease Center, Chicago, IL
Introduction: Upadacitinib (UPA) is an oral Janus kinase inhibitor (JAKi) used to treat inflammatory bowel disease (IBD). Prior phase 3 clinical trials of UPA in IBD identified 2.5% to 7.4% of patients with liver enzyme elevations (defined as ALT 3X the upper limit of normal [ULN]). The frequency of mild liver enzyme elevations is not reported. We assessed the incidence of elevated liver enzymes in a real-world population of patients with IBD treated with UPA and explore patient- and disease-related risk factors for an elevated ALT.
Methods: This retrospective cohort study included patients with IBD at the University of Chicago who were treated for 1 year with UPA. Statistical difference between ALT at different time points compared to baseline (pre-UPA initiation) was done with Wilcoxon matched-pairs signed rank test. Spearman’s correlation was used to examine the relationship between liver enzyme elevation (defined as ALT >35 U/L), inflammatory markers (C-reactive protein [CRP], fecal calprotectin [FCP]) and metabolic risk factors (hypertension, dyslipidemia, metabolic dysfunction-associated steatotic liver disease [MASLD], and elevated BMI).
Results: 160 patients (50% female, median age 37) were included. Of these, 58.1% had UC, 35% had CD, 3.1% IBD unclassified, and 4.4% pouchitis with CD-like features. Elevated ALT in 15/151 (9.9%) at baseline and 32/160 patients (20%) within the first year after UPA initiation, with 3 patients (1.9%) having ALT > 3x ULN. Compared to baseline, ALT was significantly elevated at weeks 4 (p=0.0003), 8 (p=0.002) and 24 (p=0.002), but not week 52 (p=0.234) (Figure 1). Significant correlations were found between ALT elevation and BMI over 25 kg/m2 (r=0.256, p=0.001), HTN (r=0.209, p=0.008), DLD (r=0.315, p< 0.0001), and MASLD (r=0.258, p=0.001), and male gender (0.156, p=0.049). Cigarette smoking was not associated with ALT elevation (Table I). Correlation between ALT, CRP, and FCP at different time points was not associated with changes in ALT levels other than CRP at week 8 and FCP at month 6. No patients developed liver failure or had features of Hy’s law.
Discussion: Patients with IBD experienced mild elevations in ALT after UPA initiation, particularly among those with additional metabolic risk factors. These findings emphasize the importance of careful monitoring of these patients to mitigate liver-related adverse effects. Future prospective studies should further delineate the mechanisms and long-term implications of liver enzyme elevations in this population.

Figure: Figure 1. ALT (U/L) per time-point (Baseline, week 4, week 8, week 24, week 52) after UPA initiation. Elevation seen at weeks 4 (p=0.0003), 8 (p=0.002) and 24 (p=0.002), but not week 52 (p=0.234).
Note: The table for this abstract can be viewed in the ePoster Gallery section of the ACG 2024 ePoster Site or in The American Journal of Gastroenterology's abstract supplement issue, both of which will be available starting October 27, 2024.
Disclosures:
Jeremy Klein indicated no relevant financial relationships.
Joelle St-Pierre indicated no relevant financial relationships.
Natalie Choi indicated no relevant financial relationships.
Nicole Garcia indicated no relevant financial relationships.
Emma Picker indicated no relevant financial relationships.
David Rubin: AbbVie – Consultant. AltruBio – Consultant. Apex – Consultant. Avalo Therapeutics – Consultant. Bausch Health – Consultant. Bristol Myers Squibb – Consultant. Buhlmann Diagnostics Corp – Consultant. Celgene – Consultant. ClostraBio – Consultant. Connect BioPharma – Consultant. Cornerstones Health – Board of Directors. Crohn's & Colitis Foundation – Board of Trustees. Douglas Therapeutics – Consultant. Eli Lilly – Consultant. InDex Pharmaceuticals – Consultant. Intouch Group – Consultant. Iterative Health – Consultant. Janssen Pharmaceuticals – Consultant. Odyssey Thera – Consultant. Pfizer – Consultant. Prometheus Biosciences – Consultant. Samsung Neurologica – Consultant. Takeda – Consultant, Grant/Research Support.
Jeremy A. Klein, MD1, Joelle St-Pierre, MD, PhD2, Natalie K. Choi, BA2, Nicole Garcia, BA2, Emma A. Picker, BA2, David T.. Rubin, MD, FACG2. P4400 - Elevation of Liver Enzymes In Patients With Inflammatory Bowel Disease Treated With Upadacitinib Is Associated With Metabolic Risk Factors, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
