Tuesday Poster Session
Category: IBD
P4407 - Clinical Conundrum: Recurrent Delayed Immune Checkpoint Inhibitor-Induced Colitis or de novo Inflammatory Bowel Disease (IBD)?
Tuesday, October 29, 2024
10:30 AM - 4:00 PM ET
Location: Exhibit Hall E
Has Audio
- JK
Jeffrey Kwan, MD
Walter Reed National Military Medical Center
Bethesda, MD
Presenting Author(s)
Jeffrey Kwan, MD1, Michael A. Sikes, DO2, Brett Sadowski, MD2
1Walter Reed National Military Medical Center, Bethesda, MD; 2Naval Medical Center San Diego, San Diego, CA
Introduction: The advent of immune checkpoint inhibitors (ICI) has transformed the treatment of advanced malignancies, but their immune-related adverse events (irAEs) are especially prevalent. One such irAE is ICI colitis that may develop during treatment but has been defined after discontinuation of ICI as a delayed immune-related adverse event. There are no clear differences endoscopically or clinically, and symptoms mirror IBD, though treatment is often induction with steroids plus or minus either vedolizumab or infliximab as with IBD.
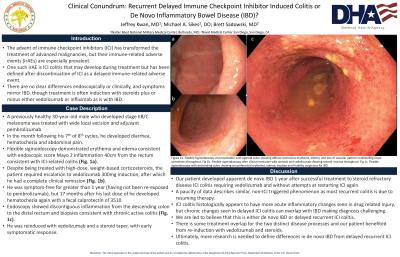
Case Description/Methods: A previously healthy 30-year-old male developed stage IIB/C melanoma which was treated wide local excision and treated with adjuvant pembrolizumab. In the month following his 7th of 8th cycles, he developed diarrhea, hematochezia and abdominal pain. Flexible sigmoidoscopy demonstrated erythema and edema consistent with endoscopic scored Mayo 2 inflammation 40cm from the rectum consistent with ICI-related colitis (Fig. 1a). Despite being treated with high-dose, weight-based corticosteroids, the patient required escalation to vedolizumab 300mg induction, after which he had a complete clinical remission (Fig. 1b). He was symptom-free for greater than 1 year (having not been re-exposed to pembrolizumab), but 17 months after his last dose of he developed hematochezia again with a fecal calprotectin of 3510. Endoscopy showed discontinuous inflammation from the descending colon to the distal rectum and biopsies consistent with chronic active colitis (Fig. 1c). He was reinduced with vedolizumab and a steroid taper, with early symptomatic response.
Discussion: Our patient developed apparent de novo IBD 1 year after successful treatment to steroid refractory disease ICI colitis requiring vedolizumab and without attempts at restarting ICI again. There is a paucity of data which describes a similar, non-ICI triggered phenomenon as most recurrent colitis is due to resuming therapy. Typically, ICI colitis histologically appears to have more acute inflammatory changes seen in drug related injury, but delayed ICI colitis can overlap with IBD and demonstrate chronic changes making diagnosis challenging. However, we are led to believe that this is either de novo IBD or delayed recurrent ICI colitis. There is some treatment overlap for the two distinct disease processes and our patient benefited from re-induction with vedolizumab and steroids. Ultimately, more research is needed to define differences in de novo IBD from delayed recurrent ICI colitis.

Disclosures:
Jeffrey Kwan, MD1, Michael A. Sikes, DO2, Brett Sadowski, MD2. P4407 - Clinical Conundrum: Recurrent Delayed Immune Checkpoint Inhibitor-Induced Colitis or de novo Inflammatory Bowel Disease (IBD)?, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
1Walter Reed National Military Medical Center, Bethesda, MD; 2Naval Medical Center San Diego, San Diego, CA
Introduction: The advent of immune checkpoint inhibitors (ICI) has transformed the treatment of advanced malignancies, but their immune-related adverse events (irAEs) are especially prevalent. One such irAE is ICI colitis that may develop during treatment but has been defined after discontinuation of ICI as a delayed immune-related adverse event. There are no clear differences endoscopically or clinically, and symptoms mirror IBD, though treatment is often induction with steroids plus or minus either vedolizumab or infliximab as with IBD.
Case Description/Methods: A previously healthy 30-year-old male developed stage IIB/C melanoma which was treated wide local excision and treated with adjuvant pembrolizumab. In the month following his 7th of 8th cycles, he developed diarrhea, hematochezia and abdominal pain. Flexible sigmoidoscopy demonstrated erythema and edema consistent with endoscopic scored Mayo 2 inflammation 40cm from the rectum consistent with ICI-related colitis (Fig. 1a). Despite being treated with high-dose, weight-based corticosteroids, the patient required escalation to vedolizumab 300mg induction, after which he had a complete clinical remission (Fig. 1b). He was symptom-free for greater than 1 year (having not been re-exposed to pembrolizumab), but 17 months after his last dose of he developed hematochezia again with a fecal calprotectin of 3510. Endoscopy showed discontinuous inflammation from the descending colon to the distal rectum and biopsies consistent with chronic active colitis (Fig. 1c). He was reinduced with vedolizumab and a steroid taper, with early symptomatic response.
Discussion: Our patient developed apparent de novo IBD 1 year after successful treatment to steroid refractory disease ICI colitis requiring vedolizumab and without attempts at restarting ICI again. There is a paucity of data which describes a similar, non-ICI triggered phenomenon as most recurrent colitis is due to resuming therapy. Typically, ICI colitis histologically appears to have more acute inflammatory changes seen in drug related injury, but delayed ICI colitis can overlap with IBD and demonstrate chronic changes making diagnosis challenging. However, we are led to believe that this is either de novo IBD or delayed recurrent ICI colitis. There is some treatment overlap for the two distinct disease processes and our patient benefited from re-induction with vedolizumab and steroids. Ultimately, more research is needed to define differences in de novo IBD from delayed recurrent ICI colitis.

Figure: Figure 1a. Flexible sigmoidoscopy at presentation with sigmoid colon showing diffuse continuous erythema, edema, and loss of vascular pattern nonbleeding linear ulcerations throughout. Fig 1b. Flexible sigmoidoscopy after clinical remission with steroids and vedolizumab showing normal mucosa throughout. Fig 1c. Flexible sigmoidoscopy with descending colon showing circumferential erythema, edema, exudate and friability suspicious for IBD.
Disclosures:
Jeffrey Kwan indicated no relevant financial relationships.
Michael Sikes indicated no relevant financial relationships.
Brett Sadowski indicated no relevant financial relationships.
Jeffrey Kwan, MD1, Michael A. Sikes, DO2, Brett Sadowski, MD2. P4407 - Clinical Conundrum: Recurrent Delayed Immune Checkpoint Inhibitor-Induced Colitis or de novo Inflammatory Bowel Disease (IBD)?, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
