Tuesday Poster Session
Category: Interventional Endoscopy
P4483 - Comparison of Diagnostic Performance of Macroscopic Onsite Evaluation (MOSE) for Tissue Acquisition With EUS-Guided Fine Needle Specimens With Rapid Onsite Evaluation (ROSE) and Conventional Techniques: A Meta-Analysis
Tuesday, October 29, 2024
10:30 AM - 4:00 PM ET
Location: Exhibit Hall E

- FJ
Fouad Jaber, MD, MS
University of Missouri - Kansas City School of Medicine
Kansas City, MO
Presenting Author(s)
Fouad Jaber, MD, MS1, Saqr Alsakarneh, MD1, Mohammad Jaber, MD2, Tala Alsharaeh, MD3, Ahmed-Jordan Salahat, MD3, Ahmed Fares, MD4, Yazan Sallam, MD5, Mohammad Adam, MD5, Anas Alselek, MD6, Mohammed Ayyad, MD7, Manesh Kumar Gangwani, MD8, Raed Darwish, MD9, Laith Numan, MD, MS10, Mohammad Bilal, MD11, Shifa Umar, MD12
1University of Missouri - Kansas City School of Medicine, Kansas City, MO; 2Al-Azhar University, Gaza, Palestinian Territories; 3The University of Jordan, Amman, 'Amman, Jordan; 4Tufts Medical Center, Boston, MA; 5University of Missouri, Kansas City, MO; 6Cairo University School of Medicine, Cairo, Al Jizah, Egypt; 7Rutgers New Jersey Medical School, Newark, NJ; 8University of Toledo, Toledo, OH; 9Ain Shams University, Cairo, Al Qahirah, Egypt; 10Saint Louis University, St. Louis, MO; 11University of Minnesota and Minneapolis VA Health Care System, Minneapolis, MN; 12Baylor College of Medicine, Houston, TX
Introduction: Endoscopic ultrasound-guided tissue acquisition (EUS-TA) is vital for the diagnosis of several mass lesions. Rapid onsite evaluation (ROSE) by a cytopathologist during EUS-TA enhances diagnostic efficacy but may not always be available. As an alternative, macroscopic onsite evaluation (MOSE) involves visually assessing the adequacy of core tissue obtained. This meta-analysis aimed to assess the diagnostic performance of MOSE and compare it with ROSE, and conventional techniques.
Methods: A systematic search of all databases until February 2024 identified studies assessing EUS-TA using MOSE was performed. Primary outcomes included sensitivity, specificity, and diagnostic accuracy, while secondary outcomes were positive predictive value (PPV), negative predictive value (NPV), and diagnostic yield. Subgroup analysis comparing MOSE with ROSE and conventional techniques was performed. Conventional technique was defined as onsite histological evaluation by the endoscopist while the solid material is sent for formal histological analysis. Pooled analysis utilized random effects models, and heterogeneity was interpreted using I2 statistics.
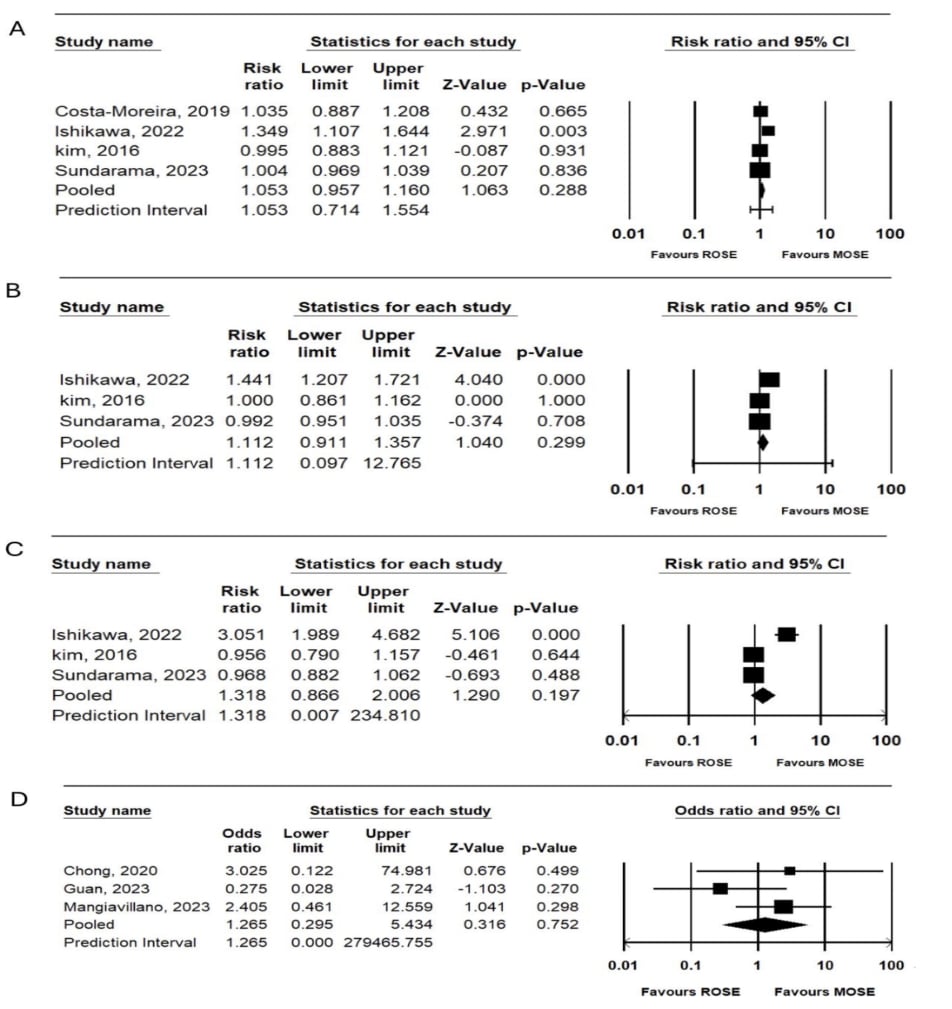
Results: Thirty-two studies, including 3826 patients, were analyzed using a random-effects model and showed that MOSE had a pooled sensitivity, specificity, and accuracy of 90.3%, 97.2%, and 90.9%, respectively. The pooled diagnostic yield, PPV, and NPV were 91.3%, 98.2%, and 65.5%. In the subset comparing MOSE with ROSE (618 patients) there was no significant difference in terms of diagnostic efficacy and safety profile. The analysis showed no significant difference between MOSE and ROSE in terms of accuracy (RR: 1.05 with 95% CI, 0.957-1.16; p 0.288), sensitivity (RR: 1.11 with 95% CI, 0.911-1.357; p 0.299), and NPV (RR: 2.026 with 95% CI, 0.266-15.42; p 0.495). There was no significant difference between the two arms in the reported associated adverse events (RR: 1.265 with 95% CI, 0.295-5.434; p 0.752). Compared to conventional methods (829 patients), MOSE outperformed with higher accuracy (92.2% vs. 88.9%,), sensitivity (91.4% vs. 87.7%), and specificity (98% vs, 97.6%). Diagnostic yield (93.2% vs. 92.6%), PPV (98.2% vs. 98.1%), and NPV (77.5% vs. 75.4%) were comparable with no significant difference.
Discussion: Our analysis suggests that MOSE can be effectively used during EUS-TA, particularly in settings where ROSE is not feasible due to cost and efficiency considerations or if expertise are not available.
Note: The table for this abstract can be viewed in the ePoster Gallery section of the ACG 2024 ePoster Site or in The American Journal of Gastroenterology's abstract supplement issue, both of which will be available starting October 27, 2024.
Disclosures:
Fouad Jaber, MD, MS1, Saqr Alsakarneh, MD1, Mohammad Jaber, MD2, Tala Alsharaeh, MD3, Ahmed-Jordan Salahat, MD3, Ahmed Fares, MD4, Yazan Sallam, MD5, Mohammad Adam, MD5, Anas Alselek, MD6, Mohammed Ayyad, MD7, Manesh Kumar Gangwani, MD8, Raed Darwish, MD9, Laith Numan, MD, MS10, Mohammad Bilal, MD11, Shifa Umar, MD12. P4483 - Comparison of Diagnostic Performance of Macroscopic Onsite Evaluation (MOSE) for Tissue Acquisition With EUS-Guided Fine Needle Specimens With Rapid Onsite Evaluation (ROSE) and Conventional Techniques: A Meta-Analysis, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
1University of Missouri - Kansas City School of Medicine, Kansas City, MO; 2Al-Azhar University, Gaza, Palestinian Territories; 3The University of Jordan, Amman, 'Amman, Jordan; 4Tufts Medical Center, Boston, MA; 5University of Missouri, Kansas City, MO; 6Cairo University School of Medicine, Cairo, Al Jizah, Egypt; 7Rutgers New Jersey Medical School, Newark, NJ; 8University of Toledo, Toledo, OH; 9Ain Shams University, Cairo, Al Qahirah, Egypt; 10Saint Louis University, St. Louis, MO; 11University of Minnesota and Minneapolis VA Health Care System, Minneapolis, MN; 12Baylor College of Medicine, Houston, TX
Introduction: Endoscopic ultrasound-guided tissue acquisition (EUS-TA) is vital for the diagnosis of several mass lesions. Rapid onsite evaluation (ROSE) by a cytopathologist during EUS-TA enhances diagnostic efficacy but may not always be available. As an alternative, macroscopic onsite evaluation (MOSE) involves visually assessing the adequacy of core tissue obtained. This meta-analysis aimed to assess the diagnostic performance of MOSE and compare it with ROSE, and conventional techniques.
Methods: A systematic search of all databases until February 2024 identified studies assessing EUS-TA using MOSE was performed. Primary outcomes included sensitivity, specificity, and diagnostic accuracy, while secondary outcomes were positive predictive value (PPV), negative predictive value (NPV), and diagnostic yield. Subgroup analysis comparing MOSE with ROSE and conventional techniques was performed. Conventional technique was defined as onsite histological evaluation by the endoscopist while the solid material is sent for formal histological analysis. Pooled analysis utilized random effects models, and heterogeneity was interpreted using I2 statistics.
Results: Thirty-two studies, including 3826 patients, were analyzed using a random-effects model and showed that MOSE had a pooled sensitivity, specificity, and accuracy of 90.3%, 97.2%, and 90.9%, respectively. The pooled diagnostic yield, PPV, and NPV were 91.3%, 98.2%, and 65.5%. In the subset comparing MOSE with ROSE (618 patients) there was no significant difference in terms of diagnostic efficacy and safety profile. The analysis showed no significant difference between MOSE and ROSE in terms of accuracy (RR: 1.05 with 95% CI, 0.957-1.16; p 0.288), sensitivity (RR: 1.11 with 95% CI, 0.911-1.357; p 0.299), and NPV (RR: 2.026 with 95% CI, 0.266-15.42; p 0.495). There was no significant difference between the two arms in the reported associated adverse events (RR: 1.265 with 95% CI, 0.295-5.434; p 0.752). Compared to conventional methods (829 patients), MOSE outperformed with higher accuracy (92.2% vs. 88.9%,), sensitivity (91.4% vs. 87.7%), and specificity (98% vs, 97.6%). Diagnostic yield (93.2% vs. 92.6%), PPV (98.2% vs. 98.1%), and NPV (77.5% vs. 75.4%) were comparable with no significant difference.
Discussion: Our analysis suggests that MOSE can be effectively used during EUS-TA, particularly in settings where ROSE is not feasible due to cost and efficiency considerations or if expertise are not available.

Figure: Figure 1: MOSE versus ROSE forest plots showing pooled A. Accuracy. B. Sensitivity. C. NPV.
Note: The table for this abstract can be viewed in the ePoster Gallery section of the ACG 2024 ePoster Site or in The American Journal of Gastroenterology's abstract supplement issue, both of which will be available starting October 27, 2024.
Disclosures:
Fouad Jaber indicated no relevant financial relationships.
Saqr Alsakarneh indicated no relevant financial relationships.
Mohammad Jaber indicated no relevant financial relationships.
Tala Alsharaeh indicated no relevant financial relationships.
Ahmed-Jordan Salahat indicated no relevant financial relationships.
Ahmed Fares indicated no relevant financial relationships.
Yazan Sallam indicated no relevant financial relationships.
Mohammad Adam indicated no relevant financial relationships.
Anas Alselek indicated no relevant financial relationships.
Mohammed Ayyad indicated no relevant financial relationships.
Manesh Kumar Gangwani indicated no relevant financial relationships.
Raed Darwish indicated no relevant financial relationships.
Laith Numan indicated no relevant financial relationships.
Mohammad Bilal: Boston Scientific – Consultant. Cook endoscopy – Speakers Bureau.
Shifa Umar indicated no relevant financial relationships.
Fouad Jaber, MD, MS1, Saqr Alsakarneh, MD1, Mohammad Jaber, MD2, Tala Alsharaeh, MD3, Ahmed-Jordan Salahat, MD3, Ahmed Fares, MD4, Yazan Sallam, MD5, Mohammad Adam, MD5, Anas Alselek, MD6, Mohammed Ayyad, MD7, Manesh Kumar Gangwani, MD8, Raed Darwish, MD9, Laith Numan, MD, MS10, Mohammad Bilal, MD11, Shifa Umar, MD12. P4483 - Comparison of Diagnostic Performance of Macroscopic Onsite Evaluation (MOSE) for Tissue Acquisition With EUS-Guided Fine Needle Specimens With Rapid Onsite Evaluation (ROSE) and Conventional Techniques: A Meta-Analysis, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
