Tuesday Poster Session
Category: Interventional Endoscopy
P4501 - Novel Artificial Intelligence (AI) Systems in Detecting Adenomas in Colonoscopy: A Systematic Review and Network Meta-Analysis
Tuesday, October 29, 2024
10:30 AM - 4:00 PM ET
Location: Exhibit Hall E

Has Audio

Muhammad YN Chaudhary, MBChB
Indiana University Southwest
Evansville, IN
Presenting Author(s)
Muhammad YN. Chaudhary, MBChB1, Mohammad Jawwad, MBBS2, Muhammad Ismail, MD3, Muhammad H N. Chaudhary, MBChB4, Fariha Hasan, MD5, Rahul Rai, MBBS6, Muhammad Sohaib Khan, 7, Monazza Riaz, MBBS8, Abdul Arham, MD9, Medha Rajamanuri, MBBS10, Umna Naveed, MBChB, MBA, MPH11, Oluwagbenga Serrano, MD, FACG12, Mohammed Barawi, MD13
1Indiana University Southwest, Evansville, IN; 2Dow Medical College, Karachi, Sindh, Pakistan; 3Indiana University Southwest, Cedar Rapids, IA; 4Manchester University NHS Foundation Trust, Stockport, England, United Kingdom; 5Cooper University Hospital, Philadelphia, PA; 6Liaquat University of Medical and Health Science, Hyderabad, Sindh, Pakistan; 7DOW University of Health Sciences, Karachi, Sindh, Pakistan; 8Dow Medical College, Norwich, England, United Kingdom; 9Baystate Medical Center, Chicopee, MA; 10Southern Illinois University, Springfield, IL; 11NHS, Liverpool, England, United Kingdom; 12Good Samaritan Hospital, Vincennes, IN; 13Ascension St. John Hospital, Detroit, MI
Introduction: Colorectal cancer is a major cause of cancer-related mortality globally. Early detection and removal of adenomatous polyps via colonoscopy are crucial in reducing its incidence and mortality. Traditional colonoscopy faces limitations in adenoma detection rates (ADRs) and adenomas per colonoscopy (APC) due to operator variability. AI-assisted colonoscopy has emerged to enhance ADRs and improve patient outcomes. This study compares the efficacy of various AI-assisted colonoscopy systems through randomized controlled trials (RCTs) to provide a comprehensive performance analysis.
Methods: Embase, Medline, and Cochrane Library databases up to March, 2024, identified RCTs comparing AI-assisted and conventional colonoscopy for detecting colorectal neoplasia. The AI-assisted systems evaluated include GI-Genius, CAD-EYE, Endoangel, Endoscreener, and EndoAID. Primary outcomes were ADR and APC, with data aggregated using a random effects model to calculate pooled odds ratios (OR) and mean difference (MD) with 95% confidence intervals (CI). The Surface under the Cumulative Ranking Curve (SUCRA) assessed relative efficacy, and subgroup analyses evaluated heterogeneity, using STATA software.
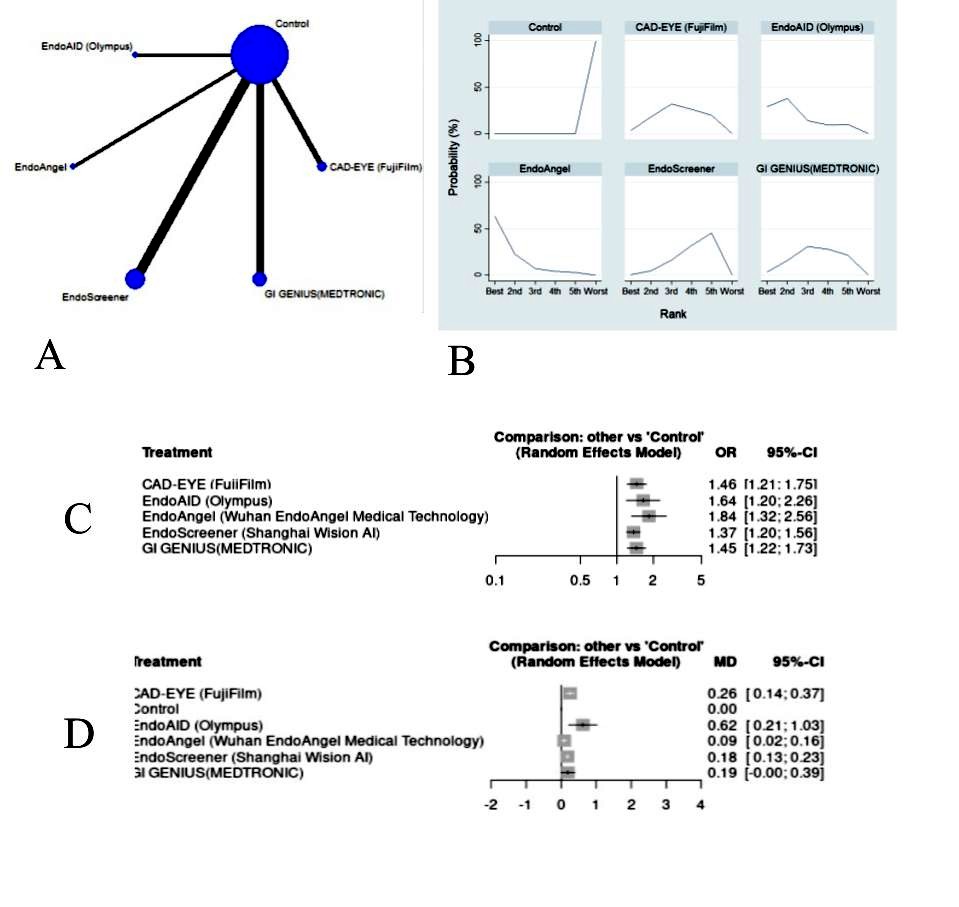
Results: Seventeen eligible RCTs with 10,547 participants were included. For ADR, EndoAngel was the most effective (OR 1.84; 95% CI, 1.32-2.56; SUCRA 0.9), followed by EndoAID (OR 1.64; 95% CI, 1.20-2.26; SUCRA 0.7). CAD-EYE and GI-Genius were jointly ranked third (OR 1.46; 95% CI, 1.21-1.75; SUCRA 0.5) and (OR 1.45; 95% CI, 1.22-1.73; SUCRA 0.5) respectively. Endoscreener ranked just above the control (OR 1.37; 95% CI, 1.20-1.56; SUCRA 0.4). GI-Genius showed higher study heterogeneity (I² = 67%). For APC, EndoAID was most effective (MD 0.62; 95% CI, 0.21-1.03), while EndoAngel was just above the control (MD 0.09; 95% CI, 0.02-0.16). Figure 1 summarizes the results
Discussion: AI-assisted colonoscopy represents a significant advancement in gastrointestinal diagnostics. Our meta-analysis identifies EndoAngel and EndoAID as the most effective systems for adenoma detection, reducing variability and enhancing procedure quality. The heterogeneity in the GI-Genius group highlights the need for standardized protocols and uniform training.
While promising, AI systems require further refinement and clinical validation. Our findings support adopting AI-assisted colonoscopy, particularly EndoAngel, to improve ADRs and patient outcomes. Future research should focus on long-term impacts and AI enhancements.
Disclosures:
Muhammad YN. Chaudhary, MBChB1, Mohammad Jawwad, MBBS2, Muhammad Ismail, MD3, Muhammad H N. Chaudhary, MBChB4, Fariha Hasan, MD5, Rahul Rai, MBBS6, Muhammad Sohaib Khan, 7, Monazza Riaz, MBBS8, Abdul Arham, MD9, Medha Rajamanuri, MBBS10, Umna Naveed, MBChB, MBA, MPH11, Oluwagbenga Serrano, MD, FACG12, Mohammed Barawi, MD13. P4501 - Novel Artificial Intelligence (AI) Systems in Detecting Adenomas in Colonoscopy: A Systematic Review and Network Meta-Analysis, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
1Indiana University Southwest, Evansville, IN; 2Dow Medical College, Karachi, Sindh, Pakistan; 3Indiana University Southwest, Cedar Rapids, IA; 4Manchester University NHS Foundation Trust, Stockport, England, United Kingdom; 5Cooper University Hospital, Philadelphia, PA; 6Liaquat University of Medical and Health Science, Hyderabad, Sindh, Pakistan; 7DOW University of Health Sciences, Karachi, Sindh, Pakistan; 8Dow Medical College, Norwich, England, United Kingdom; 9Baystate Medical Center, Chicopee, MA; 10Southern Illinois University, Springfield, IL; 11NHS, Liverpool, England, United Kingdom; 12Good Samaritan Hospital, Vincennes, IN; 13Ascension St. John Hospital, Detroit, MI
Introduction: Colorectal cancer is a major cause of cancer-related mortality globally. Early detection and removal of adenomatous polyps via colonoscopy are crucial in reducing its incidence and mortality. Traditional colonoscopy faces limitations in adenoma detection rates (ADRs) and adenomas per colonoscopy (APC) due to operator variability. AI-assisted colonoscopy has emerged to enhance ADRs and improve patient outcomes. This study compares the efficacy of various AI-assisted colonoscopy systems through randomized controlled trials (RCTs) to provide a comprehensive performance analysis.
Methods: Embase, Medline, and Cochrane Library databases up to March, 2024, identified RCTs comparing AI-assisted and conventional colonoscopy for detecting colorectal neoplasia. The AI-assisted systems evaluated include GI-Genius, CAD-EYE, Endoangel, Endoscreener, and EndoAID. Primary outcomes were ADR and APC, with data aggregated using a random effects model to calculate pooled odds ratios (OR) and mean difference (MD) with 95% confidence intervals (CI). The Surface under the Cumulative Ranking Curve (SUCRA) assessed relative efficacy, and subgroup analyses evaluated heterogeneity, using STATA software.
Results: Seventeen eligible RCTs with 10,547 participants were included. For ADR, EndoAngel was the most effective (OR 1.84; 95% CI, 1.32-2.56; SUCRA 0.9), followed by EndoAID (OR 1.64; 95% CI, 1.20-2.26; SUCRA 0.7). CAD-EYE and GI-Genius were jointly ranked third (OR 1.46; 95% CI, 1.21-1.75; SUCRA 0.5) and (OR 1.45; 95% CI, 1.22-1.73; SUCRA 0.5) respectively. Endoscreener ranked just above the control (OR 1.37; 95% CI, 1.20-1.56; SUCRA 0.4). GI-Genius showed higher study heterogeneity (I² = 67%). For APC, EndoAID was most effective (MD 0.62; 95% CI, 0.21-1.03), while EndoAngel was just above the control (MD 0.09; 95% CI, 0.02-0.16). Figure 1 summarizes the results
Discussion: AI-assisted colonoscopy represents a significant advancement in gastrointestinal diagnostics. Our meta-analysis identifies EndoAngel and EndoAID as the most effective systems for adenoma detection, reducing variability and enhancing procedure quality. The heterogeneity in the GI-Genius group highlights the need for standardized protocols and uniform training.
While promising, AI systems require further refinement and clinical validation. Our findings support adopting AI-assisted colonoscopy, particularly EndoAngel, to improve ADRs and patient outcomes. Future research should focus on long-term impacts and AI enhancements.

Figure: Figure 1: (A) Network diagram of the total number of patients analyzed in each treatment arm. The line width is proportional to the number of studies comparing each pair of interventions, and the size of each node is proportional to the number of participants (sample size). (B) Rankogram plot showing probability of each treatment (AI system) to hold a particular rank. (C) Forest plot of indirect comparison of different pairs of various AI systems through a network meta-analysis in assessing Adenoma Detection Rate (ADR). OR Odds Ratio, CI confidence interval. (D) Forest plot of indirect comparison of different pairs of various AI systems through a network meta-analysis in assessing Adenoma per colonoscopy (APC). MD Mean difference, 95% CI confidence interval.
Disclosures:
Muhammad Chaudhary indicated no relevant financial relationships.
Mohammad Jawwad indicated no relevant financial relationships.
Muhammad Ismail indicated no relevant financial relationships.
Muhammad Chaudhary indicated no relevant financial relationships.
Fariha Hasan indicated no relevant financial relationships.
Rahul Rai indicated no relevant financial relationships.
Muhammad Sohaib Khan indicated no relevant financial relationships.
Monazza Riaz indicated no relevant financial relationships.
Abdul Arham indicated no relevant financial relationships.
Medha Rajamanuri indicated no relevant financial relationships.
Umna Naveed indicated no relevant financial relationships.
Oluwagbenga Serrano: MERCK – Stock-publicly held company(excluding mutual/index funds).
Mohammed Barawi: Abbvie – Speakers Bureau. Boston scietific – Consultant. Gilead – Speakers Bureau. Olympus – Consultant.
Muhammad YN. Chaudhary, MBChB1, Mohammad Jawwad, MBBS2, Muhammad Ismail, MD3, Muhammad H N. Chaudhary, MBChB4, Fariha Hasan, MD5, Rahul Rai, MBBS6, Muhammad Sohaib Khan, 7, Monazza Riaz, MBBS8, Abdul Arham, MD9, Medha Rajamanuri, MBBS10, Umna Naveed, MBChB, MBA, MPH11, Oluwagbenga Serrano, MD, FACG12, Mohammed Barawi, MD13. P4501 - Novel Artificial Intelligence (AI) Systems in Detecting Adenomas in Colonoscopy: A Systematic Review and Network Meta-Analysis, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
