Tuesday Poster Session
Category: Interventional Endoscopy
P4503 - Comparing Diagnostic Performance of 19-Gauge and 22-Gauge Needles in Endoscopic Ultrasound-Guided Liver Biopsy: A Systematic Review and Meta-Analysis
Tuesday, October 29, 2024
10:30 AM - 4:00 PM ET
Location: Exhibit Hall E
Has Audio

Muhammad Ali Butt, MD
Allegheny General Hospital
Pittsburgh, PA
Presenting Author(s)
Muhammad Ali Butt, MD1, Sheza Malik, MD2, Rahul Karna, MD3, Taimur Muzammil, MD1, Sahla Hammad, MD4, Nabeeha Mohy-ud-din, MD5, Mohammad Bilal, MD6
1Allegheny General Hospital, Pittsburgh, PA; 2Rochester General Hospital, Rochester, NY; 3University of Minnesota Medical Center, Minneapolis, MN; 4Atrium Health, Charlotte, NC; 5Allegheny Health Network, Pittsburgh, PA; 6University of Minnesota and Minneapolis VA Health Care System, Minneapolis, MN
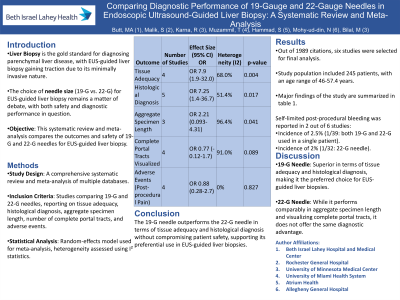
Introduction: Liver biopsy is the gold standard for the diagnosis and management of parenchymal liver disease. Over the last decade, endoscopic ultrasound (EUS)-guided liver biopsy has been gaining popularity as well. However, the optimal needle size to perform EUS guided liver biopsy safely is unclear. We aimed to perform a systematic review and meta-analysis comparing the outcomes and safety of the two commonly used needle sizes, 19-gauge (G) and 22-G needles, for EUS-guided liver biopsy.
Methods: A comprehensive search of multiple databases was conducted to identify studies comparing 19-G and 22-G fine needle biopsy (FNB) needles for liver biopsy. Studies reporting tissue adequacy, histologic diagnosis, aggregate specimen length, number of complete portal tracts visualized, and adverse events were included. Meta-analysis was conducted using random-effects models, and heterogeneity was assessed using standard methods.
Results: Out of 1989 citations, six studies were selected for final analysis. The study population included 245 patients, with an age range of 46-57.4 years. The 19-G needle was superior in terms of tissue adequacy (OR 7.9, 95% CI 1.9-32.0, p=0.004) and obtaining a histological diagnosis (OR 7.25, 95% CI 1.4-36.7, p=0.017) compared to the 22-G needles. Both needle sizes performed similarly in aggregate specimen length (OR 2.21, 95% CI 0.093-4.31, p=0.041) and the number of complete portal tracts visualized (OR 0.77, 95% CI -0.12-1.7, p=0.089). No statistically significant difference was found in post-procedural pain between the two needle sizes (OR 0.88, 95% CI 0.28-2.7, p=0.827). Self-limited post-procedural bleeding was reported in 2 out of 6 studies, with incidences of 2.5% (1/39: both 19-G and 22-G used in a single patient) and 2% (1/32: 22-G needle).
Discussion: Our meta-analysis supports the preferential use of 19-G needles over 22-G needles for EUS-LB, given their superior performance in tissue adequacy and histological diagnosis, with no difference in post-procedural adverse events.
Note: The table for this abstract can be viewed in the ePoster Gallery section of the ACG 2024 ePoster Site or in The American Journal of Gastroenterology's abstract supplement issue, both of which will be available starting October 27, 2024.
Disclosures:
Muhammad Ali Butt, MD1, Sheza Malik, MD2, Rahul Karna, MD3, Taimur Muzammil, MD1, Sahla Hammad, MD4, Nabeeha Mohy-ud-din, MD5, Mohammad Bilal, MD6. P4503 - Comparing Diagnostic Performance of 19-Gauge and 22-Gauge Needles in Endoscopic Ultrasound-Guided Liver Biopsy: A Systematic Review and Meta-Analysis, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
1Allegheny General Hospital, Pittsburgh, PA; 2Rochester General Hospital, Rochester, NY; 3University of Minnesota Medical Center, Minneapolis, MN; 4Atrium Health, Charlotte, NC; 5Allegheny Health Network, Pittsburgh, PA; 6University of Minnesota and Minneapolis VA Health Care System, Minneapolis, MN
Introduction: Liver biopsy is the gold standard for the diagnosis and management of parenchymal liver disease. Over the last decade, endoscopic ultrasound (EUS)-guided liver biopsy has been gaining popularity as well. However, the optimal needle size to perform EUS guided liver biopsy safely is unclear. We aimed to perform a systematic review and meta-analysis comparing the outcomes and safety of the two commonly used needle sizes, 19-gauge (G) and 22-G needles, for EUS-guided liver biopsy.
Methods: A comprehensive search of multiple databases was conducted to identify studies comparing 19-G and 22-G fine needle biopsy (FNB) needles for liver biopsy. Studies reporting tissue adequacy, histologic diagnosis, aggregate specimen length, number of complete portal tracts visualized, and adverse events were included. Meta-analysis was conducted using random-effects models, and heterogeneity was assessed using standard methods.
Results: Out of 1989 citations, six studies were selected for final analysis. The study population included 245 patients, with an age range of 46-57.4 years. The 19-G needle was superior in terms of tissue adequacy (OR 7.9, 95% CI 1.9-32.0, p=0.004) and obtaining a histological diagnosis (OR 7.25, 95% CI 1.4-36.7, p=0.017) compared to the 22-G needles. Both needle sizes performed similarly in aggregate specimen length (OR 2.21, 95% CI 0.093-4.31, p=0.041) and the number of complete portal tracts visualized (OR 0.77, 95% CI -0.12-1.7, p=0.089). No statistically significant difference was found in post-procedural pain between the two needle sizes (OR 0.88, 95% CI 0.28-2.7, p=0.827). Self-limited post-procedural bleeding was reported in 2 out of 6 studies, with incidences of 2.5% (1/39: both 19-G and 22-G used in a single patient) and 2% (1/32: 22-G needle).
Discussion: Our meta-analysis supports the preferential use of 19-G needles over 22-G needles for EUS-LB, given their superior performance in tissue adequacy and histological diagnosis, with no difference in post-procedural adverse events.
Note: The table for this abstract can be viewed in the ePoster Gallery section of the ACG 2024 ePoster Site or in The American Journal of Gastroenterology's abstract supplement issue, both of which will be available starting October 27, 2024.
Disclosures:
Muhammad Ali Butt indicated no relevant financial relationships.
Sheza Malik indicated no relevant financial relationships.
Rahul Karna indicated no relevant financial relationships.
Taimur Muzammil indicated no relevant financial relationships.
Sahla Hammad indicated no relevant financial relationships.
Nabeeha Mohy-ud-din indicated no relevant financial relationships.
Mohammad Bilal: Boston Scientific – Consultant. Cook endoscopy – Speakers Bureau.
Muhammad Ali Butt, MD1, Sheza Malik, MD2, Rahul Karna, MD3, Taimur Muzammil, MD1, Sahla Hammad, MD4, Nabeeha Mohy-ud-din, MD5, Mohammad Bilal, MD6. P4503 - Comparing Diagnostic Performance of 19-Gauge and 22-Gauge Needles in Endoscopic Ultrasound-Guided Liver Biopsy: A Systematic Review and Meta-Analysis, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.

