Tuesday Poster Session
Category: Liver
P4565 - The Utilization of Non-Invasive Tests (NITs) for the Clinical Management of Metabolic Dysfunction Associated Steatotic Liver Disease (MASLD) in the United States
Tuesday, October 29, 2024
10:30 AM - 4:00 PM ET
Location: Exhibit Hall E

Has Audio

Alina Allen, MD
Mayo Clinic
Rochester, MN
Presenting Author(s)
Alina Allen, MD1, Naim Alkhouri, MD2, Mazen Nouredden, MD, MHSc3, Vincent Wai-Sun Wong, MD4, Emmanuel Tsochatzis, MD5, Leyla deAvila, BA6, Andrei Racila, MS6, Maria M. Stepanova, PhD6, Fatema Nader, MBA6, Henry Mark, MA6, Jeffrey Lazarus, PhD7, Laurent Castera, MD8, Zobair Younossi, MD, MPH, FACG6
1Mayo Clinic, Rochester, MN; 2Arizona Liver Health, Chandler, AZ; 3Houston Methodist Hospital, Houston, TX; 4The Chinese University of Hong Kong, Hong Kong, Hong Kong; 5Royal Free Hospital and UCL, London, England, United Kingdom; 6The Global NASH Council, Washington, DC; 7Barcelona Institute for Global Health (ISGlobal), Hospital Clinic, University of Barcelona, Barcelona, Madrid, Spain; 8Universite Paris-Cite, Beaujon Hospital, Clichy, Franche-Comte, France
Introduction: NITs are used to risk-stratify MASLD patients. The aim was to survey the use of NITs among U.S. providers who care for patients with MASLD.
Methods: The 38-item survey was designed by members of The Global NASH Council. This ongoing global survey has been completed by 208 providers from 35 countries with 80 from the US. Providers were asked about MASLD risks for advanced fibrosis, which NITs (and what cutoff values) they use to rule out and rule in significant (F2-F4) and advanced (F3-F4) fibrosis, how they prefer to monitor patients.
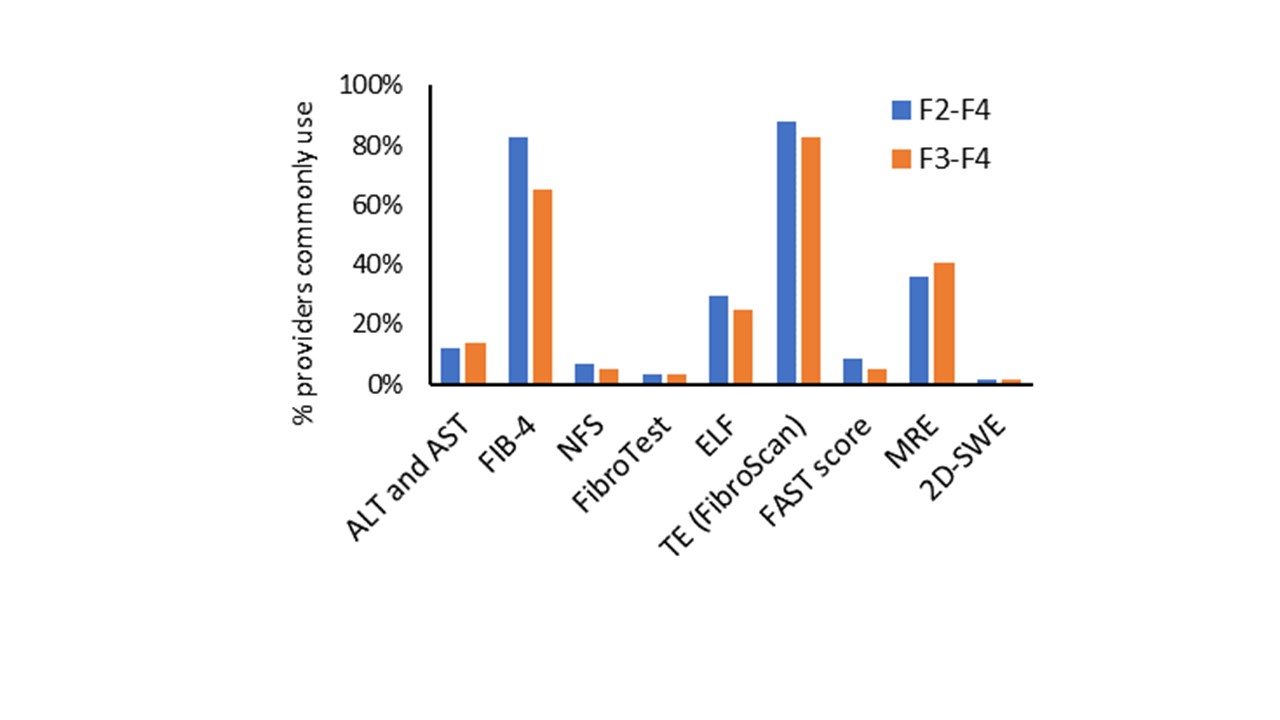
Results: Of the U.S. providers who responded to date, 79% were MD and 19% were advanced practice providers, 88% had gastro-hepatology specialty, 44% had ≥20 years of practice experience, 53% worked in an academic setting, and 69% saw ≥20 MASLD patients per month. Of the respondents, 51% would use NITs to risk-stratify all MASLD patients while 87% would do the same for patients with type 2 diabetes (T2D), obesity (79%), abnormal liver enzymes (76%), steatosis on imaging (72%), or dyslipidemia (71%). Among those responding to NIT questions, transient elastography (TE) was available to 88%, FIB-4 to 83%, MRE to 36%, and ELF to 29%; other NITs (NFS, FibroTest, FAST, SWE, Agile 3+, Agile-4) to 3-24% in their clinical practices. Among the NITs most commonly used for ruling out significant or advanced fibrosis, TE, FIB-4, MRE and ELF were the top 4 options (Figure). The most commonly used cutoffs for ruling out significant fibrosis were TE< 8 kPa (43%), FIB-4 < 1.30 for age < 65 (63%), FIB-4< 2.00 for age ≥65 (42%), and MRE < 3.14 kPa (43%). For ruling in advanced fibrosis in MASLD, the most common thresholds were TE ≥10 kPa (19%), FIB-4 ≥2.67 (14%), TE ≥12 kPa (12%), or MRE ≥3.6 kPa (11%). To diagnose advance fibrosis, 56% of respondents would use 2 NITs (FIB-4 followed by TE, MRE or ELF), while 23% would consider one NIT as sufficient and 11% would confirm with liver biopsy. Only 5% would use a different than the standard cutoff for patients with T2D. Most (56%) providers would rarely (< 10%) refer their patients for biopsy. Most commonly used method for monitoring disease progression was TE ( >70%), and most providers ( >66%) would monitor patients at intermediate or high risk every year. Finally, 66% follow AASLD MASLD guideline recommendation regarding NITs.
Discussion: Despite good awareness of NITs for MASLD in the U.S., there is some variability in the NITs and their thresholds in clinical practice.
Disclosures:
Alina Allen, MD1, Naim Alkhouri, MD2, Mazen Nouredden, MD, MHSc3, Vincent Wai-Sun Wong, MD4, Emmanuel Tsochatzis, MD5, Leyla deAvila, BA6, Andrei Racila, MS6, Maria M. Stepanova, PhD6, Fatema Nader, MBA6, Henry Mark, MA6, Jeffrey Lazarus, PhD7, Laurent Castera, MD8, Zobair Younossi, MD, MPH, FACG6. P4565 - The Utilization of Non-Invasive Tests (NITs) for the Clinical Management of Metabolic Dysfunction Associated Steatotic Liver Disease (MASLD) in the United States, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
1Mayo Clinic, Rochester, MN; 2Arizona Liver Health, Chandler, AZ; 3Houston Methodist Hospital, Houston, TX; 4The Chinese University of Hong Kong, Hong Kong, Hong Kong; 5Royal Free Hospital and UCL, London, England, United Kingdom; 6The Global NASH Council, Washington, DC; 7Barcelona Institute for Global Health (ISGlobal), Hospital Clinic, University of Barcelona, Barcelona, Madrid, Spain; 8Universite Paris-Cite, Beaujon Hospital, Clichy, Franche-Comte, France
Introduction: NITs are used to risk-stratify MASLD patients. The aim was to survey the use of NITs among U.S. providers who care for patients with MASLD.
Methods: The 38-item survey was designed by members of The Global NASH Council. This ongoing global survey has been completed by 208 providers from 35 countries with 80 from the US. Providers were asked about MASLD risks for advanced fibrosis, which NITs (and what cutoff values) they use to rule out and rule in significant (F2-F4) and advanced (F3-F4) fibrosis, how they prefer to monitor patients.
Results: Of the U.S. providers who responded to date, 79% were MD and 19% were advanced practice providers, 88% had gastro-hepatology specialty, 44% had ≥20 years of practice experience, 53% worked in an academic setting, and 69% saw ≥20 MASLD patients per month. Of the respondents, 51% would use NITs to risk-stratify all MASLD patients while 87% would do the same for patients with type 2 diabetes (T2D), obesity (79%), abnormal liver enzymes (76%), steatosis on imaging (72%), or dyslipidemia (71%). Among those responding to NIT questions, transient elastography (TE) was available to 88%, FIB-4 to 83%, MRE to 36%, and ELF to 29%; other NITs (NFS, FibroTest, FAST, SWE, Agile 3+, Agile-4) to 3-24% in their clinical practices. Among the NITs most commonly used for ruling out significant or advanced fibrosis, TE, FIB-4, MRE and ELF were the top 4 options (Figure). The most commonly used cutoffs for ruling out significant fibrosis were TE< 8 kPa (43%), FIB-4 < 1.30 for age < 65 (63%), FIB-4< 2.00 for age ≥65 (42%), and MRE < 3.14 kPa (43%). For ruling in advanced fibrosis in MASLD, the most common thresholds were TE ≥10 kPa (19%), FIB-4 ≥2.67 (14%), TE ≥12 kPa (12%), or MRE ≥3.6 kPa (11%). To diagnose advance fibrosis, 56% of respondents would use 2 NITs (FIB-4 followed by TE, MRE or ELF), while 23% would consider one NIT as sufficient and 11% would confirm with liver biopsy. Only 5% would use a different than the standard cutoff for patients with T2D. Most (56%) providers would rarely (< 10%) refer their patients for biopsy. Most commonly used method for monitoring disease progression was TE ( >70%), and most providers ( >66%) would monitor patients at intermediate or high risk every year. Finally, 66% follow AASLD MASLD guideline recommendation regarding NITs.
Discussion: Despite good awareness of NITs for MASLD in the U.S., there is some variability in the NITs and their thresholds in clinical practice.

Figure: Figure
Disclosures:
Alina Allen indicated no relevant financial relationships.
Naim Alkhouri: Altimmune, Chronwell ,Cytodyne,Madrigal Pharmaceuticals,Merck,Novo Nordisk,Rivus,Takeda,Terns – Consultant, Grant/Research Support.
Mazen Nouredden: Altimmune – Advisor or Review Panel Member. Chronwell – Stock Options. Cytodyne – Stock Options. Madrigal Pharmaceuticals – Advisor or Review Panel Member, Consultant. Merck – Advisor or Review Panel Member. Novo Nordisk – Advisor or Review Panel Member. Rivus – Stock Options. Takeda – Advisor or Review Panel Member. Terns – Advisor or Review Panel Member.
Vincent Wai-Sun Wong indicated no relevant financial relationships.
Emmanuel Tsochatzis indicated no relevant financial relationships.
Leyla deAvila indicated no relevant financial relationships.
Andrei Racila indicated no relevant financial relationships.
Maria Stepanova indicated no relevant financial relationships.
Fatema Nader indicated no relevant financial relationships.
Henry Mark indicated no relevant financial relationships.
Jeffrey Lazarus indicated no relevant financial relationships.
Laurent Castera indicated no relevant financial relationships.
Zobair Younossi: Intercept, Cymabay, Boehringer Ingelheim, Ipsen, BMS, GSK, NovoNordisk, Merck and Abbott. – Grant/Research Support. Madrigal – Grant/Research Support. Siemens – Grant/Research Support.
Alina Allen, MD1, Naim Alkhouri, MD2, Mazen Nouredden, MD, MHSc3, Vincent Wai-Sun Wong, MD4, Emmanuel Tsochatzis, MD5, Leyla deAvila, BA6, Andrei Racila, MS6, Maria M. Stepanova, PhD6, Fatema Nader, MBA6, Henry Mark, MA6, Jeffrey Lazarus, PhD7, Laurent Castera, MD8, Zobair Younossi, MD, MPH, FACG6. P4565 - The Utilization of Non-Invasive Tests (NITs) for the Clinical Management of Metabolic Dysfunction Associated Steatotic Liver Disease (MASLD) in the United States, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
