Tuesday Poster Session
Category: Liver
P4591 - Trends in the Early Adoption of Terlipressin Among Hospitalized Adults with Hepatorenal Syndrome in the U.S.: A Real-World Analysis
Tuesday, October 29, 2024
10:30 AM - 4:00 PM ET
Location: Exhibit Hall E
Has Audio

Robert Wong, MD, MS, FACG
Stanford/VA Palo Alto
Palo Alto, CA
Presenting Author(s)
Robert Wong, MD, MS, FACG1, Andrew S. Allegretti, MD, MSc2, Xingyue Huang, PhD3, Mary P. Panaccio, PhD, MS3, Sanaz Cardoza, PharmD3, John Niewoehner, PharmD3, Rahul Rajkumar, MD, MPH4, Jonathan Lilley, BA5, Kunal Lodaya, MD5
1Stanford/VA Palo Alto, Palo Alto, CA; 2Massachusetts General Hospital, Boston, MA; 3Mallinckrodt Pharmaceuticals, Bridgewater, NJ; 4Boston Strategic Partners, Boston, MA; 5Boston Strategic Partners, Philadelphia, PA
Introduction: Hepatorenal syndrome-acute kidney injury (HRS-AKI) is a serious complication of decompensated liver cirrhosis, leading to significant morbidity and mortality. Terlipressin is the only FDA-approved treatment for adults with HRS and is a preferred therapy according to national and international guidelines. We evaluated real-world trends and outcomes in hospitalized U.S. patients with HRS treated with terlipressin.
Methods: A HIPAA-compliant, hospital chargemaster database (Premier), was queried to identify patients hospitalized with HRS and treated with terlipressin for ≥ 2 days between Sep 2022 - May 2024. HRS diagnoses and terlipressin administration were identified using ICD-10-CM/PCS, National Drug Codes, and billing codes. Socio-demographics, clinical characteristics, terlipressin treatment patterns, and clinical outcomes were evaluated.
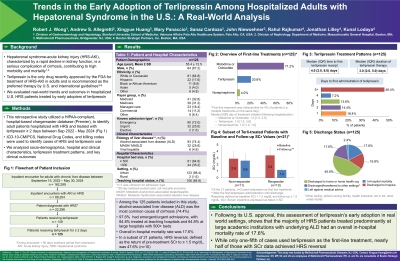
Results: A total of 125 patients were included. Mean age was 55.4y ± 12.5, 67.2% were male, 64.8% were White (non-Hispanic), 17.6% were Hispanic, and 8.8% were Black/African American (non-Hispanic). Nearly all patients had an index hospitalization classified as emergent/urgent (97.6%), 84.8% sought care from teaching hospitals, and 64.8% were treated at large hospitals with 500+ beds. The most common etiology of cirrhosis was alcohol-related liver disease (ALD, 74.4%). On average (SD), terlipressin was initiated on day 6 (6.7) of hospitalization, and only 20.8% (n= 26) received terlipressin as 1st line treatment; 78.4% were treated first with midodrine or octreotide (53.6% received both), and 11.2% with norepinephrine (not mutually exclusive). Average (SD) terlipressin treatment duration was 4.8 (4.8) days. Based on a subset of n= 21 with serum creatinine (SCr) measurements and a baseline SCr > 1.5 mg/dL at admission (mean [SD]: 3.3 [1.1]), HRS reversal, defined as the return of pre-treatment SCr to ≤ 1.5 mg/dL, was seen in n= 10 (47.6%), with a mean (SD) post-treatment SCr of 1.2 (0.2) mg/dL. Overall, 45.6% were discharged to home or a home health organization. In-hospital mortality was 17.6%, and 16.8% were discharged to hospice care. The median (IQR) length of hospitalization was 15 days (9 – 25).
Discussion: In the first post-approval evaluation of terlipressin real-world treatment and outcomes in the U.S., the majority of HRS patients were treated at large academic centers and had underlying ALD. Overall mortality was 17.6%, and while terlipressin was used 1st line in only 20.8% of patients, 47.6% of patients with SCr data achieved HRS reversal.
Note: The table for this abstract can be viewed in the ePoster Gallery section of the ACG 2024 ePoster Site or in The American Journal of Gastroenterology's abstract supplement issue, both of which will be available starting October 27, 2024.
Disclosures:
Robert Wong, MD, MS, FACG1, Andrew S. Allegretti, MD, MSc2, Xingyue Huang, PhD3, Mary P. Panaccio, PhD, MS3, Sanaz Cardoza, PharmD3, John Niewoehner, PharmD3, Rahul Rajkumar, MD, MPH4, Jonathan Lilley, BA5, Kunal Lodaya, MD5. P4591 - Trends in the Early Adoption of Terlipressin Among Hospitalized Adults with Hepatorenal Syndrome in the U.S.: A Real-World Analysis, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
1Stanford/VA Palo Alto, Palo Alto, CA; 2Massachusetts General Hospital, Boston, MA; 3Mallinckrodt Pharmaceuticals, Bridgewater, NJ; 4Boston Strategic Partners, Boston, MA; 5Boston Strategic Partners, Philadelphia, PA
Introduction: Hepatorenal syndrome-acute kidney injury (HRS-AKI) is a serious complication of decompensated liver cirrhosis, leading to significant morbidity and mortality. Terlipressin is the only FDA-approved treatment for adults with HRS and is a preferred therapy according to national and international guidelines. We evaluated real-world trends and outcomes in hospitalized U.S. patients with HRS treated with terlipressin.
Methods: A HIPAA-compliant, hospital chargemaster database (Premier), was queried to identify patients hospitalized with HRS and treated with terlipressin for ≥ 2 days between Sep 2022 - May 2024. HRS diagnoses and terlipressin administration were identified using ICD-10-CM/PCS, National Drug Codes, and billing codes. Socio-demographics, clinical characteristics, terlipressin treatment patterns, and clinical outcomes were evaluated.
Results: A total of 125 patients were included. Mean age was 55.4y ± 12.5, 67.2% were male, 64.8% were White (non-Hispanic), 17.6% were Hispanic, and 8.8% were Black/African American (non-Hispanic). Nearly all patients had an index hospitalization classified as emergent/urgent (97.6%), 84.8% sought care from teaching hospitals, and 64.8% were treated at large hospitals with 500+ beds. The most common etiology of cirrhosis was alcohol-related liver disease (ALD, 74.4%). On average (SD), terlipressin was initiated on day 6 (6.7) of hospitalization, and only 20.8% (n= 26) received terlipressin as 1st line treatment; 78.4% were treated first with midodrine or octreotide (53.6% received both), and 11.2% with norepinephrine (not mutually exclusive). Average (SD) terlipressin treatment duration was 4.8 (4.8) days. Based on a subset of n= 21 with serum creatinine (SCr) measurements and a baseline SCr > 1.5 mg/dL at admission (mean [SD]: 3.3 [1.1]), HRS reversal, defined as the return of pre-treatment SCr to ≤ 1.5 mg/dL, was seen in n= 10 (47.6%), with a mean (SD) post-treatment SCr of 1.2 (0.2) mg/dL. Overall, 45.6% were discharged to home or a home health organization. In-hospital mortality was 17.6%, and 16.8% were discharged to hospice care. The median (IQR) length of hospitalization was 15 days (9 – 25).
Discussion: In the first post-approval evaluation of terlipressin real-world treatment and outcomes in the U.S., the majority of HRS patients were treated at large academic centers and had underlying ALD. Overall mortality was 17.6%, and while terlipressin was used 1st line in only 20.8% of patients, 47.6% of patients with SCr data achieved HRS reversal.
Note: The table for this abstract can be viewed in the ePoster Gallery section of the ACG 2024 ePoster Site or in The American Journal of Gastroenterology's abstract supplement issue, both of which will be available starting October 27, 2024.
Disclosures:
Robert Wong: Durect Corporation – Grant/Research Support. Exact Sciences – Grant/Research Support. Gilead Sciences – Grant/Research Support. Thera Technologies – Grant/Research Support.
Andrew Allegretti: Bioporto – Consultant. Mallinkcrodt Pharmaceuticals – Consultant. Motric Bio – Consultant. Ocelot Bio – Consultant. Sequana Medical – Consultant.
Xingyue Huang: Mallinckrodt Pharmaceuticals – Employee.
Mary Panaccio indicated no relevant financial relationships.
Sanaz Cardoza: Mallinckrodt Pharmaceuticals – Employee, Stock-publicly held company(excluding mutual/index funds).
John Niewoehner: Mallinckrodt Pharmaceuticals – Employee.
Rahul Rajkumar: Mallinckrodt Pharmaceuticals (MNK) – Consultant.
Jonathan Lilley: Mallinckrodt – Consultant.
Kunal Lodaya: Mallinckrodt – Consultant.
Robert Wong, MD, MS, FACG1, Andrew S. Allegretti, MD, MSc2, Xingyue Huang, PhD3, Mary P. Panaccio, PhD, MS3, Sanaz Cardoza, PharmD3, John Niewoehner, PharmD3, Rahul Rajkumar, MD, MPH4, Jonathan Lilley, BA5, Kunal Lodaya, MD5. P4591 - Trends in the Early Adoption of Terlipressin Among Hospitalized Adults with Hepatorenal Syndrome in the U.S.: A Real-World Analysis, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.

