Tuesday Poster Session
Category: Liver
P4602 - Pre-Transplant Cardiovascular Risk Stratification and Early Post-transplant Cardiac Events in Liver Transplant Recipients
Tuesday, October 29, 2024
10:30 AM - 4:00 PM ET
Location: Exhibit Hall E
Has Audio

Sandra Au, MD
Duke University Medical Center
Durham, NC
Presenting Author(s)
Sandra Au, MD1, Ashley Jowell, MD1, Alice Parish, 2, Carolyn Lekavich, PhD, ANPC3, Patricia Gorgone, BSN4, Kerry Higuera, 4, Shwetha Dash, 4, Donna Niedzwiecki, PhD2, Michael Blazing, MD4, Lindsay Y. King, MD4, Kara Wegermann, MD4
1Duke University Medical Center, Durham, NC; 2Duke University, Durham, NC; 3Duke University School of Medicine, Durham, NC; 4Duke University Health System, Durham, NC
Introduction: Cardiovascular events (CVE) are a major cause of morbidity and mortality within the first year of liver transplantation (LT), impacting 15-30% of recipients. Pre-transplant CV risk evaluation strategies differ among transplant centers. Accurately forecasting post-LT CVE is imperative for refining evaluations and optimizing CV outcomes.
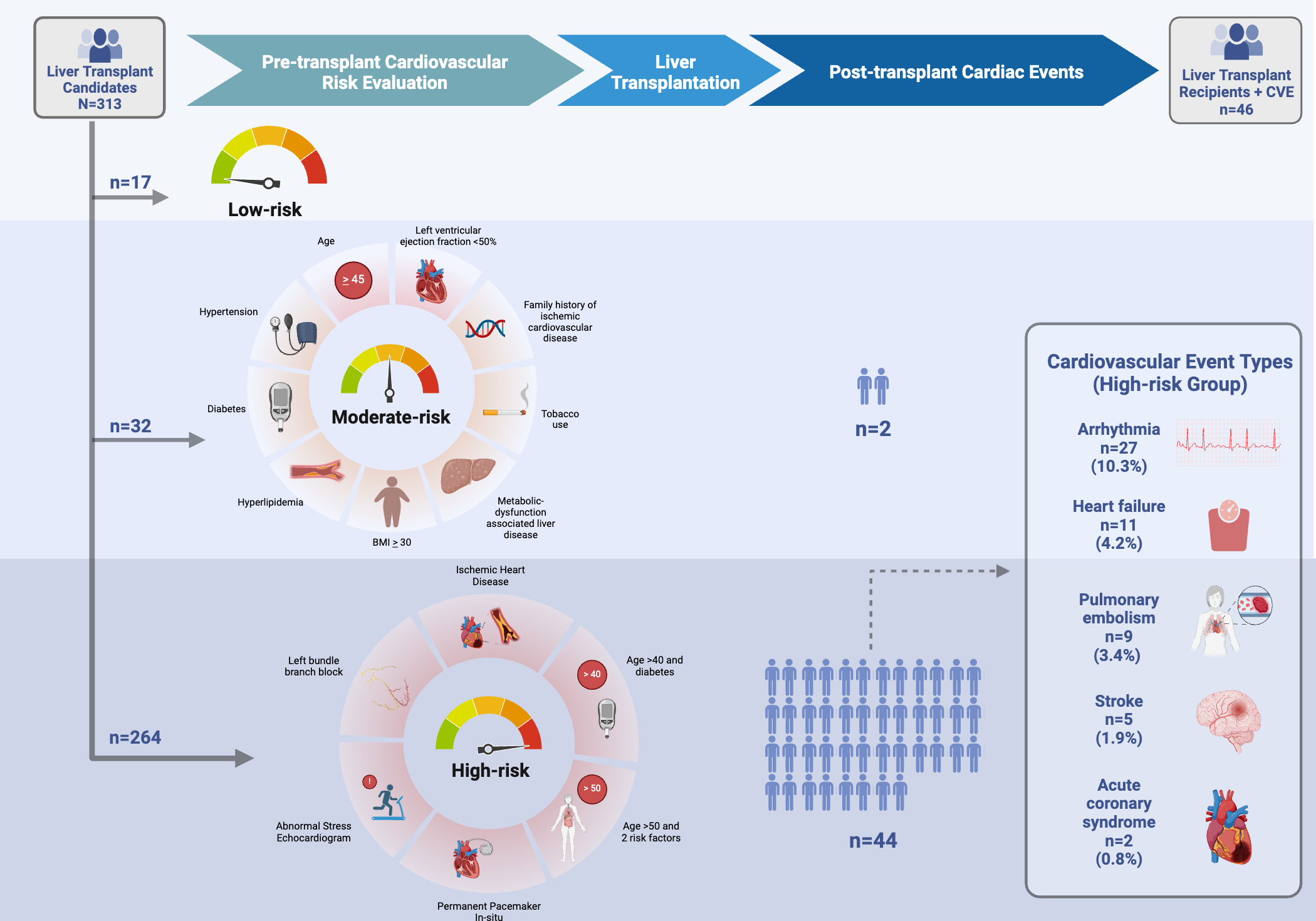
Methods: We retrospectively assessed all adult LT recipients (LTR) including simultaneous liver-kidney recipients at our center from 7/1/2019 to 6/30/2022. Demographic, comorbidity, and CV testing data were obtained via an institutional transplant database and used to define low, moderate, and high-risk groups based on institutional protocol (Figure). The primary outcome was 1-year post-LT nonfatal CVE, including arrhythmias, pulmonary embolism, acute coronary syndrome, heart failure, or stroke, and was manually adjudicated using chart review. Mortality rates were monitored as a competing risk. Descriptive analyses were performed.
Results: A total of 313 LTR were included, comprising 17 low-risk (5.4%), 32 moderate-risk (10.2%) and 264 high-risk (84.3%) (Table). As expected, high-risk LTR were older (median age at evaluation in years: low-risk 34 [IQR: 32, 41], moderate-risk 43 [37, 51.5], high-risk 59 [53.0, 65.0]). More high-risk LTR were former smokers. Most high-risk LTR had MASLD (34%) or alcohol-associated liver disease (ALD, 25%), most moderate-risk LTR had ALD (31%) or cholestatic liver disease (28%), and most low-risk LTR had ALD (47%). Among 46 (14.7%) unique patients that experienced a CVE, 96% (54/57) were in the high-risk group, and none were low-risk. In high-risk LTR, the median time-to-event was 10 days [IQR: 4, 55], the most common CVE was arrhythmia (10%), and most rare acute coronary syndrome (0.8%) (Figure). 16 (6%) of high-risk patients, and 4 (12.5%) of moderate-risk patients died within 1-year post-LT.
Discussion: We observed similar rates of post-LT CVE as reported in the literature, with arrhythmias being the most common. The majority of CVE occurred within the high-risk group, suggesting our approach appropriately captures LTR at risk of early post-LT CVE. Still, most high-risk LTR had no CVE, suggesting that they may be over-classified. Moderate risk LTR had the highest mortality rate. These findings underscore the need to enhance the precision of comprehensive pre-LT CV risk stratification, and refine targeted interventions aimed at mitigating risk of post-LT cardiac complications.
Note: The table for this abstract can be viewed in the ePoster Gallery section of the ACG 2024 ePoster Site or in The American Journal of Gastroenterology's abstract supplement issue, both of which will be available starting October 27, 2024.
Disclosures:
Sandra Au, MD1, Ashley Jowell, MD1, Alice Parish, 2, Carolyn Lekavich, PhD, ANPC3, Patricia Gorgone, BSN4, Kerry Higuera, 4, Shwetha Dash, 4, Donna Niedzwiecki, PhD2, Michael Blazing, MD4, Lindsay Y. King, MD4, Kara Wegermann, MD4. P4602 - Pre-Transplant Cardiovascular Risk Stratification and Early Post-transplant Cardiac Events in Liver Transplant Recipients, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
1Duke University Medical Center, Durham, NC; 2Duke University, Durham, NC; 3Duke University School of Medicine, Durham, NC; 4Duke University Health System, Durham, NC
Introduction: Cardiovascular events (CVE) are a major cause of morbidity and mortality within the first year of liver transplantation (LT), impacting 15-30% of recipients. Pre-transplant CV risk evaluation strategies differ among transplant centers. Accurately forecasting post-LT CVE is imperative for refining evaluations and optimizing CV outcomes.
Methods: We retrospectively assessed all adult LT recipients (LTR) including simultaneous liver-kidney recipients at our center from 7/1/2019 to 6/30/2022. Demographic, comorbidity, and CV testing data were obtained via an institutional transplant database and used to define low, moderate, and high-risk groups based on institutional protocol (Figure). The primary outcome was 1-year post-LT nonfatal CVE, including arrhythmias, pulmonary embolism, acute coronary syndrome, heart failure, or stroke, and was manually adjudicated using chart review. Mortality rates were monitored as a competing risk. Descriptive analyses were performed.
Results: A total of 313 LTR were included, comprising 17 low-risk (5.4%), 32 moderate-risk (10.2%) and 264 high-risk (84.3%) (Table). As expected, high-risk LTR were older (median age at evaluation in years: low-risk 34 [IQR: 32, 41], moderate-risk 43 [37, 51.5], high-risk 59 [53.0, 65.0]). More high-risk LTR were former smokers. Most high-risk LTR had MASLD (34%) or alcohol-associated liver disease (ALD, 25%), most moderate-risk LTR had ALD (31%) or cholestatic liver disease (28%), and most low-risk LTR had ALD (47%). Among 46 (14.7%) unique patients that experienced a CVE, 96% (54/57) were in the high-risk group, and none were low-risk. In high-risk LTR, the median time-to-event was 10 days [IQR: 4, 55], the most common CVE was arrhythmia (10%), and most rare acute coronary syndrome (0.8%) (Figure). 16 (6%) of high-risk patients, and 4 (12.5%) of moderate-risk patients died within 1-year post-LT.
Discussion: We observed similar rates of post-LT CVE as reported in the literature, with arrhythmias being the most common. The majority of CVE occurred within the high-risk group, suggesting our approach appropriately captures LTR at risk of early post-LT CVE. Still, most high-risk LTR had no CVE, suggesting that they may be over-classified. Moderate risk LTR had the highest mortality rate. These findings underscore the need to enhance the precision of comprehensive pre-LT CV risk stratification, and refine targeted interventions aimed at mitigating risk of post-LT cardiac complications.

Figure: Figure. Post-transplant cardiovascular events stratified by pre-transplant cardiovascular risk groups, as defined based on institutional transplant center protocols.
1. All candidates universally undergo assessment of CV risk factors. Universal testing includes transthoracic echocardiogram and electrocardiogram. In the absence of abnormal findings, or meeting risk factor criteria, candidates are considered low-risk, and there is no further testing. Those considered moderate-risk^ underwent cardiac stress testing of some modality, and those at high-risk^^ warranted cardiology consultation and further diagnostic evaluation per the clinician’s discretion.
^Moderate-risk criteria: Age 45 years or older OR left ventricular ejection fraction <50% OR metabolic-dysfunction associated liver disease OR 2 or more risk factors for ischemic heart disease (hypertension, diabetes, hyperlipidemia, BMI >30, tobacco use, family history)
^^High-risk criteria: history of ischemic heart disease OR age greater than 40 years and diabetes OR age greater than 50 years and 2 or more risk factors OR abnormal stress echocardiogram OR left bundle branch block on electrocardiogram OR permanent pacemaker in situ.
1. All candidates universally undergo assessment of CV risk factors. Universal testing includes transthoracic echocardiogram and electrocardiogram. In the absence of abnormal findings, or meeting risk factor criteria, candidates are considered low-risk, and there is no further testing. Those considered moderate-risk^ underwent cardiac stress testing of some modality, and those at high-risk^^ warranted cardiology consultation and further diagnostic evaluation per the clinician’s discretion.
^Moderate-risk criteria: Age 45 years or older OR left ventricular ejection fraction <50% OR metabolic-dysfunction associated liver disease OR 2 or more risk factors for ischemic heart disease (hypertension, diabetes, hyperlipidemia, BMI >30, tobacco use, family history)
^^High-risk criteria: history of ischemic heart disease OR age greater than 40 years and diabetes OR age greater than 50 years and 2 or more risk factors OR abnormal stress echocardiogram OR left bundle branch block on electrocardiogram OR permanent pacemaker in situ.
Note: The table for this abstract can be viewed in the ePoster Gallery section of the ACG 2024 ePoster Site or in The American Journal of Gastroenterology's abstract supplement issue, both of which will be available starting October 27, 2024.
Disclosures:
Sandra Au indicated no relevant financial relationships.
Ashley Jowell indicated no relevant financial relationships.
Alice Parish indicated no relevant financial relationships.
Carolyn Lekavich indicated no relevant financial relationships.
Patricia Gorgone indicated no relevant financial relationships.
Kerry Higuera indicated no relevant financial relationships.
Shwetha Dash indicated no relevant financial relationships.
Donna Niedzwiecki indicated no relevant financial relationships.
Michael Blazing indicated no relevant financial relationships.
Lindsay King indicated no relevant financial relationships.
Kara Wegermann: Madrigal Pharmaceuticals – Study investigator. Mirum Pharmaceuticals – Study investigator.
Sandra Au, MD1, Ashley Jowell, MD1, Alice Parish, 2, Carolyn Lekavich, PhD, ANPC3, Patricia Gorgone, BSN4, Kerry Higuera, 4, Shwetha Dash, 4, Donna Niedzwiecki, PhD2, Michael Blazing, MD4, Lindsay Y. King, MD4, Kara Wegermann, MD4. P4602 - Pre-Transplant Cardiovascular Risk Stratification and Early Post-transplant Cardiac Events in Liver Transplant Recipients, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.

