Tuesday Poster Session
Category: Liver
P4631 - Trends in Prescriber Characteristics of Hepatitis C Direct-Acting Antivirals in the United States
Tuesday, October 29, 2024
10:30 AM - 4:00 PM ET
Location: Exhibit Hall E

Xiaohan Ying, MD
New York-Presbyterian / Weill Cornell Medical Center
New York, NY
Presenting Author(s)
Xiaohan Ying, MD1, Nicole Ng, MD1, Kristen Fadel, MD1, Russell Rosenblatt, MD1, Catherine Lucero, MD1, Arun B.. Jesudian, MD2
1New York-Presbyterian / Weill Cornell Medical Center, New York, NY; 2Weill Cornell Medicine, New York, NY
Introduction: Hepatitis C virus (HCV) infection is among the leading causes of liver disease and mortality, with over 2 million individuals in the US currently infected. The approval of direct-acting antivirals (DAAs) revolutionized its treatment. However, incidence of HCV continues to grow in the US. In this study, we aim to better understand prescriber characteristics for DAAs for patients with HCV.
Methods: Medicare part D data (2015-2021) were used to determine the number of 30-day HCV anti-viral prescriptions, number of beneficiaries, total drug spending, and prescriber characteristics. Prescriber specialties were categorized into 1. Primary Care (PCP), 2. Gastroenterology (GI), 3. Infectious Diseases (ID), 4. Advanced Practice Provider (APP), and 5. Other. Microsoft Excel was used for data analysis, and JoinPoint was used for annual percent change (APC) and trends analysis.
Results: Seven medications were included: branded and generic ledipasvir/sofosbuvir, branded and generic sofosbuvir/velpatasvir, sofosbuvir, sofosbuvir/velpatasvir/voxilaprevir, and glecaprevir/pibrentasvir.
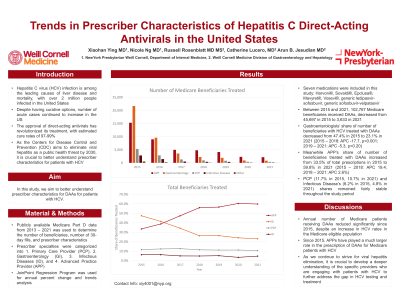
Between 2015 and 2021, 102,767 Medicare patients received DAAs. The annual number of patients receiving DAAs decreased from 45,697 in 2015 to 3,633 in 2021 (2015-2017: APC -50.7, 95% CI -57.4 to -39.4, p< 0.001; 2017-2021: APC -25.6, 95% CI -30.8 to -13.9, p< 0.001).
When evaluating by share of total patients treated, APPs experienced an increase from 33% in 2015 to 60% in 2021 (2015-2018: APC 19.4, 95% CI 17.8 to 21.4, p< 0.001; 2018-2021: APC 2.6, 95% CI 1.04 to 4.07, p=0.003). PCP and ID’s share remained stable (PCP: 11.7% in 2015 to 10.7% in 2021, APC -2.1, 95% CI -4.4 to 0.3, p=0.08; ID: 6.2% in 2015 to 4.8% in 2021, APC -6.7, 95% CI -14.0 to 1.2, p=0.10). GI’s share decreased from 47.4% in 2015 to 26.3% in 2018 (APC -17.7%, 95% CI -25.1 to -12.4, p< 0.001), after which it remained stable (APC -5.3, 95% CI -10.9 to 4.0, p=0.20).
Discussion: Since 2015, APPs have played a much larger role in the prescription of DAAs for patients with HCV. Total number of Medicare patients receiving DAAs reduced significantly. While Medicare patients have accounted for a smaller portion of acute HCV infections, we have witnessed an increase in HCV rates in the Medicare eligible population in recent years. As we continue to strive for viral hepatitis elimination, it is crucial to develop a deeper understanding of the specific providers who are engaging with patients with HCV to further address the gap in HCV testing and treatment.
Disclosures:
Xiaohan Ying, MD1, Nicole Ng, MD1, Kristen Fadel, MD1, Russell Rosenblatt, MD1, Catherine Lucero, MD1, Arun B.. Jesudian, MD2. P4631 - Trends in Prescriber Characteristics of Hepatitis C Direct-Acting Antivirals in the United States, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
1New York-Presbyterian / Weill Cornell Medical Center, New York, NY; 2Weill Cornell Medicine, New York, NY
Introduction: Hepatitis C virus (HCV) infection is among the leading causes of liver disease and mortality, with over 2 million individuals in the US currently infected. The approval of direct-acting antivirals (DAAs) revolutionized its treatment. However, incidence of HCV continues to grow in the US. In this study, we aim to better understand prescriber characteristics for DAAs for patients with HCV.
Methods: Medicare part D data (2015-2021) were used to determine the number of 30-day HCV anti-viral prescriptions, number of beneficiaries, total drug spending, and prescriber characteristics. Prescriber specialties were categorized into 1. Primary Care (PCP), 2. Gastroenterology (GI), 3. Infectious Diseases (ID), 4. Advanced Practice Provider (APP), and 5. Other. Microsoft Excel was used for data analysis, and JoinPoint was used for annual percent change (APC) and trends analysis.
Results: Seven medications were included: branded and generic ledipasvir/sofosbuvir, branded and generic sofosbuvir/velpatasvir, sofosbuvir, sofosbuvir/velpatasvir/voxilaprevir, and glecaprevir/pibrentasvir.
Between 2015 and 2021, 102,767 Medicare patients received DAAs. The annual number of patients receiving DAAs decreased from 45,697 in 2015 to 3,633 in 2021 (2015-2017: APC -50.7, 95% CI -57.4 to -39.4, p< 0.001; 2017-2021: APC -25.6, 95% CI -30.8 to -13.9, p< 0.001).
When evaluating by share of total patients treated, APPs experienced an increase from 33% in 2015 to 60% in 2021 (2015-2018: APC 19.4, 95% CI 17.8 to 21.4, p< 0.001; 2018-2021: APC 2.6, 95% CI 1.04 to 4.07, p=0.003). PCP and ID’s share remained stable (PCP: 11.7% in 2015 to 10.7% in 2021, APC -2.1, 95% CI -4.4 to 0.3, p=0.08; ID: 6.2% in 2015 to 4.8% in 2021, APC -6.7, 95% CI -14.0 to 1.2, p=0.10). GI’s share decreased from 47.4% in 2015 to 26.3% in 2018 (APC -17.7%, 95% CI -25.1 to -12.4, p< 0.001), after which it remained stable (APC -5.3, 95% CI -10.9 to 4.0, p=0.20).
Discussion: Since 2015, APPs have played a much larger role in the prescription of DAAs for patients with HCV. Total number of Medicare patients receiving DAAs reduced significantly. While Medicare patients have accounted for a smaller portion of acute HCV infections, we have witnessed an increase in HCV rates in the Medicare eligible population in recent years. As we continue to strive for viral hepatitis elimination, it is crucial to develop a deeper understanding of the specific providers who are engaging with patients with HCV to further address the gap in HCV testing and treatment.
Disclosures:
Xiaohan Ying indicated no relevant financial relationships.
Nicole Ng indicated no relevant financial relationships.
Kristen Fadel indicated no relevant financial relationships.
Russell Rosenblatt indicated no relevant financial relationships.
Catherine Lucero indicated no relevant financial relationships.
Arun Jesudian: Bausch Health – Consultant. Madrigal Pharmaceuticals – Speakers Bureau. Novo Nordisk – Advisor or Review Panel Member, Consultant. Orphalan – Advisor or Review Panel Member, Consultant. Salix Pharmaceuticals – Consultant, Speakers Bureau.
Xiaohan Ying, MD1, Nicole Ng, MD1, Kristen Fadel, MD1, Russell Rosenblatt, MD1, Catherine Lucero, MD1, Arun B.. Jesudian, MD2. P4631 - Trends in Prescriber Characteristics of Hepatitis C Direct-Acting Antivirals in the United States, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
