Tuesday Poster Session
Category: Liver
P4653 - Improving the Detection of Chronic Hepatitis C Virus Infection Using a Reflexive, Single-Sample Method for Infection Confirmation
Tuesday, October 29, 2024
10:30 AM - 4:00 PM ET
Location: Exhibit Hall E

Has Audio
- JI
John Ingram, DO
University of Mississippi Medical Center
Jackson, MS
Presenting Author(s)
John Ingram, DO, Yousef Hreish, MD, Aun Muhammad, MBBS, Benjamin Billingsley, DO, Namrata Paladugu, BS, MD, Simon Barinas, , Elizabeth Paine, MD, James Galbraith, MD
University of Mississippi Medical Center, Jackson, MS
Introduction:
Despite the availability of curative, all-oral medications, hepatitis C virus (HCV) infection remains a significant public health concern due to numerous barriers to treatment. One notable obstacle is the need to confirm HCV infection following an initial reactive HCV antibody (Ab) test, which requires a two-step process involving a second blood specimen for viral load assessment. This requirement poses challenges in settings like emergency departments (EDs), where follow-up care may be limited, particularly among high-risk populations such as the economically disadvantaged, uninsured, incarcerated individuals, and people who inject drugs (PWID). To address this issue and enhance HCV confirmation rates, we implemented a simplified method using a single blood sample method for both HCV antibody testing and confirmation.
Methods:
On March 7, 2024, our urban ED-based HCV testing program introduced a single-sample method for HCV confirmation, utilizing an electronic health record order to designate viral load testing using the same specimen tube employed for HCV antibody testing. HCV antibody testing was conducted using the Abbott ARCHITECT i1000, and viral load testing was performed using the Abbott RealTime System (Abbott Industries, Chicago, IL). To assess the effectiveness of this method, we compared outcomes from 900 consecutive HCV antibody testing encounters before (using a two-step method) and after implementing the single-sample method.
Results:
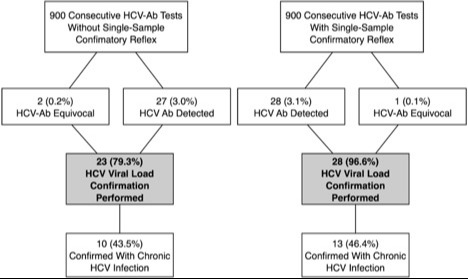
The single-sample reflex method for HCV confirmation improved confirmatory viral load performance rates from 79.3% to 96.6% (p=0.10). See Figure.
Discussion:
The single-sample reflex method demonstrated a substantial enhancement in confirmatory viral load performance, suggesting its potential to streamline HCV testing, particularly in settings like EDs where obtaining a second blood specimen is challenging. Additionally, this approach may lead to faster HCV diagnoses and reduce costs associated with additional specimen collection, care coordination, and follow-up healthcare visits. Further studies with larger sample sizes are warranted to confirm these findings and assess the method's broader impact.
Disclosures:
John Ingram, DO, Yousef Hreish, MD, Aun Muhammad, MBBS, Benjamin Billingsley, DO, Namrata Paladugu, BS, MD, Simon Barinas, , Elizabeth Paine, MD, James Galbraith, MD. P4653 - Improving the Detection of Chronic Hepatitis C Virus Infection Using a Reflexive, Single-Sample Method for Infection Confirmation, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
University of Mississippi Medical Center, Jackson, MS
Introduction:
Despite the availability of curative, all-oral medications, hepatitis C virus (HCV) infection remains a significant public health concern due to numerous barriers to treatment. One notable obstacle is the need to confirm HCV infection following an initial reactive HCV antibody (Ab) test, which requires a two-step process involving a second blood specimen for viral load assessment. This requirement poses challenges in settings like emergency departments (EDs), where follow-up care may be limited, particularly among high-risk populations such as the economically disadvantaged, uninsured, incarcerated individuals, and people who inject drugs (PWID). To address this issue and enhance HCV confirmation rates, we implemented a simplified method using a single blood sample method for both HCV antibody testing and confirmation.
Methods:
On March 7, 2024, our urban ED-based HCV testing program introduced a single-sample method for HCV confirmation, utilizing an electronic health record order to designate viral load testing using the same specimen tube employed for HCV antibody testing. HCV antibody testing was conducted using the Abbott ARCHITECT i1000, and viral load testing was performed using the Abbott RealTime System (Abbott Industries, Chicago, IL). To assess the effectiveness of this method, we compared outcomes from 900 consecutive HCV antibody testing encounters before (using a two-step method) and after implementing the single-sample method.
Results:
The single-sample reflex method for HCV confirmation improved confirmatory viral load performance rates from 79.3% to 96.6% (p=0.10). See Figure.
Discussion:
The single-sample reflex method demonstrated a substantial enhancement in confirmatory viral load performance, suggesting its potential to streamline HCV testing, particularly in settings like EDs where obtaining a second blood specimen is challenging. Additionally, this approach may lead to faster HCV diagnoses and reduce costs associated with additional specimen collection, care coordination, and follow-up healthcare visits. Further studies with larger sample sizes are warranted to confirm these findings and assess the method's broader impact.

Figure: Two-step sample without reflex method for HCV confirmation versus single-sample reflex method for HCV confirmation
Disclosures:
John Ingram indicated no relevant financial relationships.
Yousef Hreish indicated no relevant financial relationships.
Aun Muhammad indicated no relevant financial relationships.
Benjamin Billingsley indicated no relevant financial relationships.
Namrata Paladugu indicated no relevant financial relationships.
Simon Barinas indicated no relevant financial relationships.
Elizabeth Paine indicated no relevant financial relationships.
James Galbraith: Gilead Sciences – Grant/Research Support.
John Ingram, DO, Yousef Hreish, MD, Aun Muhammad, MBBS, Benjamin Billingsley, DO, Namrata Paladugu, BS, MD, Simon Barinas, , Elizabeth Paine, MD, James Galbraith, MD. P4653 - Improving the Detection of Chronic Hepatitis C Virus Infection Using a Reflexive, Single-Sample Method for Infection Confirmation, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
