Tuesday Poster Session
Category: Liver
P4861 - Drug-Induced Liver Injury Due to Green Tea Extract
Tuesday, October 29, 2024
10:30 AM - 4:00 PM ET
Location: Exhibit Hall E
Has Audio
- DJ
Danielle Jackson, DO
UT Health Science Center
Tyler, TX
Presenting Author(s)
Danielle Jackson, DO1, Muhammad Almas, MD2, Omer Chowdhury, DO3, Hyon Kang, DO1
1University of Texas Health East Texas Physicians, Tyler, TX; 2University of Texas Health Sciences Center, Tyler, TX; 3University of Texas Health Science Center, Tyler, TX
Introduction: Amongst the most common causes of drug induced liver injury (DILI) are acetaminophen, antibiotics, and antiepileptics. In recent years, herbal products, including green tea extract (GTE), have been rising in popularity due to their advertised benefit. We present a rare case of acute liver failure (ALF) caused by consumption of GTE.
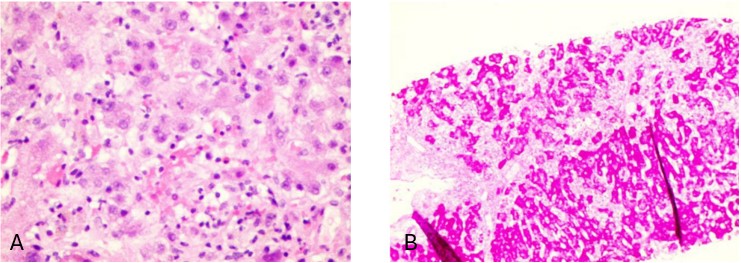
Case Description/Methods: A 59-year-old female presented to the ED for one week of ongoing diffuse abdominal pain, nausea, and jaundice. Initial labs on presentation were AST of >2,000 U/L; ALT of >3,500 U/L, platelets of 37.5 K, INR of 1.4 and total bilirubin of 6.8 mg/dL. Patient’s vital signs were stable; however, she was visibly jaundiced with presence of scleral icterus. Extensive liver workup for ALF was unremarkable including autoimmune, hepatitis, medications, and other infectious etiologies. Imaging including CT abdomen, gallbladder and liver ultrasound all demonstrated normal liver echotexture. Further investigation revealed the patient had been ingesting a weight loss supplement labeled ‘BurnXT’ with GTE as the active ingredient prior to onset of her symptoms. A liver biopsy showed significant widespread hepatocellular injury and cell death associated with acute and chronic inflammation involving all areas of liver, as shown in figures 1A and 1B. Throughout her hospitalization, patient’s synthetic liver function worsened. As such, she was transferred to a transplant capable facility for evaluation, but thankfully her liver enzymes improved and she was discharged home. Patient had a follow up appointment with her PCP one month later with complete resolution of her symptoms and return of her liver enzymes to baseline.
Discussion: Green tea extract originates from Camellia sinensis, and contains many polyphenols including catechins, which are thought to help promote weight loss. GTE has about 10% make up of catechins however the concentration is variable between supplements. Though GTE may appear safe, these supplements are not FDA regulated, and rare cases of ALF due to GTE have been reported in the literature. This case highlights the importance of bringing awareness to potential harmful herbal supplements that may cause acute liver injury. If unrecognized and left untreated, acute liver failure may occur. Thankfully, our patient made a full recovery without the need for liver transplant. It is important that healthcare providers are vigilant and incorporate a thorough history in all patients who present with acute liver injury.
Disclosures:
Danielle Jackson, DO1, Muhammad Almas, MD2, Omer Chowdhury, DO3, Hyon Kang, DO1. P4861 - Drug-Induced Liver Injury Due to Green Tea Extract, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
1University of Texas Health East Texas Physicians, Tyler, TX; 2University of Texas Health Sciences Center, Tyler, TX; 3University of Texas Health Science Center, Tyler, TX
Introduction: Amongst the most common causes of drug induced liver injury (DILI) are acetaminophen, antibiotics, and antiepileptics. In recent years, herbal products, including green tea extract (GTE), have been rising in popularity due to their advertised benefit. We present a rare case of acute liver failure (ALF) caused by consumption of GTE.
Case Description/Methods: A 59-year-old female presented to the ED for one week of ongoing diffuse abdominal pain, nausea, and jaundice. Initial labs on presentation were AST of >2,000 U/L; ALT of >3,500 U/L, platelets of 37.5 K, INR of 1.4 and total bilirubin of 6.8 mg/dL. Patient’s vital signs were stable; however, she was visibly jaundiced with presence of scleral icterus. Extensive liver workup for ALF was unremarkable including autoimmune, hepatitis, medications, and other infectious etiologies. Imaging including CT abdomen, gallbladder and liver ultrasound all demonstrated normal liver echotexture. Further investigation revealed the patient had been ingesting a weight loss supplement labeled ‘BurnXT’ with GTE as the active ingredient prior to onset of her symptoms. A liver biopsy showed significant widespread hepatocellular injury and cell death associated with acute and chronic inflammation involving all areas of liver, as shown in figures 1A and 1B. Throughout her hospitalization, patient’s synthetic liver function worsened. As such, she was transferred to a transplant capable facility for evaluation, but thankfully her liver enzymes improved and she was discharged home. Patient had a follow up appointment with her PCP one month later with complete resolution of her symptoms and return of her liver enzymes to baseline.
Discussion: Green tea extract originates from Camellia sinensis, and contains many polyphenols including catechins, which are thought to help promote weight loss. GTE has about 10% make up of catechins however the concentration is variable between supplements. Though GTE may appear safe, these supplements are not FDA regulated, and rare cases of ALF due to GTE have been reported in the literature. This case highlights the importance of bringing awareness to potential harmful herbal supplements that may cause acute liver injury. If unrecognized and left untreated, acute liver failure may occur. Thankfully, our patient made a full recovery without the need for liver transplant. It is important that healthcare providers are vigilant and incorporate a thorough history in all patients who present with acute liver injury.

Figure: Figure 1 (A): Parenchymal inflammation and feathery degeneration of hepatocytes, H&E, 400x Figure 1 (B): Positive glycogen stores in viable hepatocytes (dark pink) loss of cytoplasmic glycogen in generating hepatocytes (pale cells), PAS stain, 100x
Disclosures:
Danielle Jackson indicated no relevant financial relationships.
Muhammad Almas indicated no relevant financial relationships.
Omer Chowdhury indicated no relevant financial relationships.
Hyon Kang indicated no relevant financial relationships.
Danielle Jackson, DO1, Muhammad Almas, MD2, Omer Chowdhury, DO3, Hyon Kang, DO1. P4861 - Drug-Induced Liver Injury Due to Green Tea Extract, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
