Tuesday Poster Session
Category: Small Intestine
P4988 - Thinking Beyond the Colon: A Case of Duodenal Malignancy in a Patient With a Delayed Diagnosis of Lynch Syndrome
Tuesday, October 29, 2024
10:30 AM - 4:00 PM ET
Location: Exhibit Hall E
Has Audio
- PS
Priyanka Shah, MD
Lewis Katz School of Medicine at Temple University
Philadelphia, PA
Presenting Author(s)
Priyanka Shah, MD1, Samyuktha Manikandan, MD2, Manasa Vallabhaneni, MD2, Frank Friedenberg, MD, MS1
1Lewis Katz School of Medicine at Temple University, Philadelphia, PA; 2Temple University Hospital, Philadelphia, PA
Introduction: Lynch syndrome (LS) is the most common cause of inherited colorectal cancer (CRC). LS increases the risk of other malignancies, but only a few screening guidelines for these extracolonic malignancies exist. Here we present a case of small bowel malignancy associated with recurrent CRC in a patient with LS that highlights the need for robust screening guidelines.
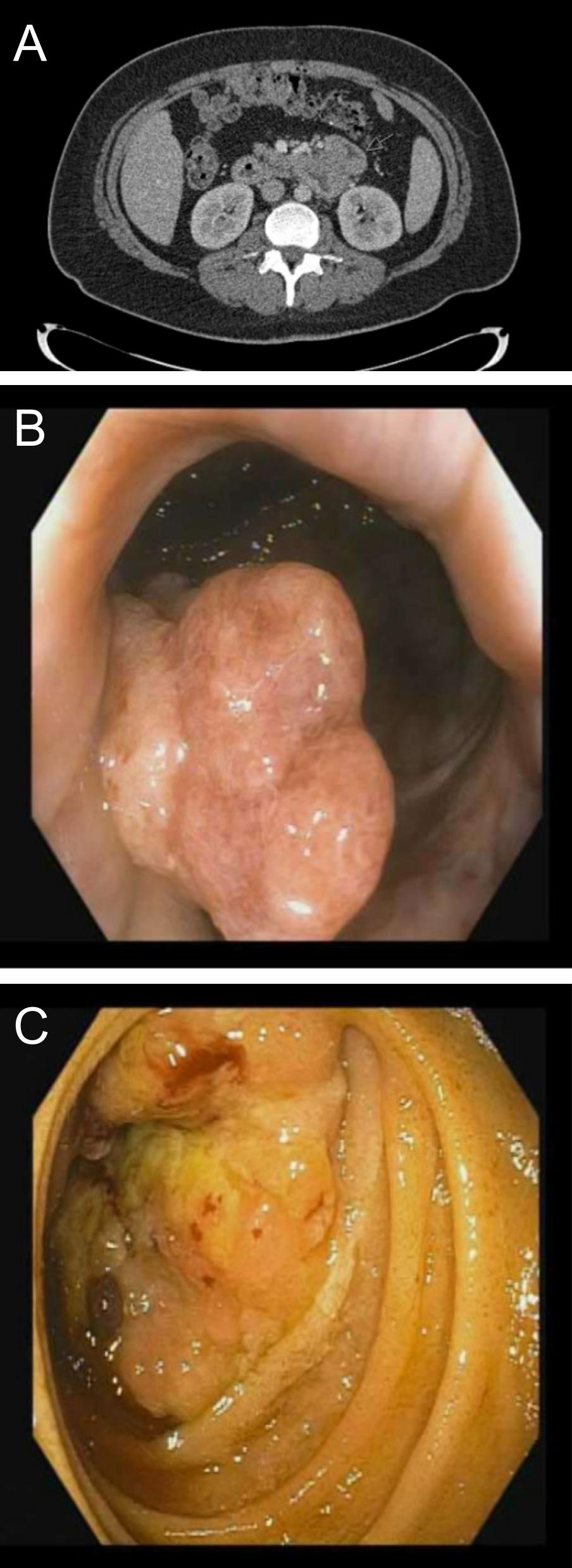
Case Description/Methods: A 34-year-old male with history of CRC at age 21 status-post surgery and chemotherapy presented with hematochezia and a hemoglobin of 5.3 g/dL. CT revealed abnormal wall thickening at the duodenojejunal junction (Fig. A), a necrotic retroperitoneal lymph node, and a cecal polyp. Colonoscopy showed a rectal mass (Fig. B); biopsy confirmed moderately-differentiated invasive adenocarcinoma. Repeat colonoscopy demonstrated a cecal polyp, with pathology showing tubulovillous adenoma with high-grade dysplasia. Upper endoscopy revealed an ulcerated, infiltrative mass of the distal duodenum (Fig. C); pathology showed adenocarcinoma. The duodenal specimen was MMR-deficient in MLH1, MSH2, MSH6, and PMS2. Genetic testing showed an MLH1 mutation, specifically c.1333C >T (p.Gln445*), consistent with LS. He started dostarlimab.
Discussion: 2015 ACG guidelines recommend considering upper endoscopy screening between ages 30 and 35 in patients at risk of or diagnosed with LS, but the quality of evidence is very low (Table 1). Risk stratification by LS mutation could lead to stronger evidence for guideline-directed screening. For example, the risk of extracolonic cancers in patients with MSH6 mutations is comparatively low, so screening could be emphasized in patients with higher risk mutations, such as in this patient with an MLH1 mutation. This case also highlights the importance of not losing high-risk patients to follow-up. This patient should have been tested for LS at age 21. If testing had been done then, the patient’s duodenal cancer may have been detected earlier, as genetic testing revealed a 0.4% to 11% risk of small bowel malignancy.
Bhattacharya P, et al. Lynch Syndrome. In: StatPearls. Treasure Island (FL): StatPearls Publishing; February 4, 2023.
Bonadona V, et al. Cancer Risks Associated With Germline Mutations in MLH1, MSH2, and MSH6 Genes in Lynch Syndrome. JAMA. 2011;305(22):2304–2310.
Syngal S, et al. ACG clinical guideline: genetic testing and management of hereditary gastrointestinal cancer syndromes. The American Journal of Gastroenterology. 2015;110(2):223.
Note: The table for this abstract can be viewed in the ePoster Gallery section of the ACG 2024 ePoster Site or in The American Journal of Gastroenterology's abstract supplement issue, both of which will be available starting October 27, 2024.
Disclosures:
Priyanka Shah, MD1, Samyuktha Manikandan, MD2, Manasa Vallabhaneni, MD2, Frank Friedenberg, MD, MS1. P4988 - Thinking Beyond the Colon: A Case of Duodenal Malignancy in a Patient With a Delayed Diagnosis of Lynch Syndrome, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
1Lewis Katz School of Medicine at Temple University, Philadelphia, PA; 2Temple University Hospital, Philadelphia, PA
Introduction: Lynch syndrome (LS) is the most common cause of inherited colorectal cancer (CRC). LS increases the risk of other malignancies, but only a few screening guidelines for these extracolonic malignancies exist. Here we present a case of small bowel malignancy associated with recurrent CRC in a patient with LS that highlights the need for robust screening guidelines.
Case Description/Methods: A 34-year-old male with history of CRC at age 21 status-post surgery and chemotherapy presented with hematochezia and a hemoglobin of 5.3 g/dL. CT revealed abnormal wall thickening at the duodenojejunal junction (Fig. A), a necrotic retroperitoneal lymph node, and a cecal polyp. Colonoscopy showed a rectal mass (Fig. B); biopsy confirmed moderately-differentiated invasive adenocarcinoma. Repeat colonoscopy demonstrated a cecal polyp, with pathology showing tubulovillous adenoma with high-grade dysplasia. Upper endoscopy revealed an ulcerated, infiltrative mass of the distal duodenum (Fig. C); pathology showed adenocarcinoma. The duodenal specimen was MMR-deficient in MLH1, MSH2, MSH6, and PMS2. Genetic testing showed an MLH1 mutation, specifically c.1333C >T (p.Gln445*), consistent with LS. He started dostarlimab.
Discussion: 2015 ACG guidelines recommend considering upper endoscopy screening between ages 30 and 35 in patients at risk of or diagnosed with LS, but the quality of evidence is very low (Table 1). Risk stratification by LS mutation could lead to stronger evidence for guideline-directed screening. For example, the risk of extracolonic cancers in patients with MSH6 mutations is comparatively low, so screening could be emphasized in patients with higher risk mutations, such as in this patient with an MLH1 mutation. This case also highlights the importance of not losing high-risk patients to follow-up. This patient should have been tested for LS at age 21. If testing had been done then, the patient’s duodenal cancer may have been detected earlier, as genetic testing revealed a 0.4% to 11% risk of small bowel malignancy.
Bhattacharya P, et al. Lynch Syndrome. In: StatPearls. Treasure Island (FL): StatPearls Publishing; February 4, 2023.
Bonadona V, et al. Cancer Risks Associated With Germline Mutations in MLH1, MSH2, and MSH6 Genes in Lynch Syndrome. JAMA. 2011;305(22):2304–2310.
Syngal S, et al. ACG clinical guideline: genetic testing and management of hereditary gastrointestinal cancer syndromes. The American Journal of Gastroenterology. 2015;110(2):223.

Figure: A: Wall thickening at duodenojejunal junction on CT
B: Rectal mass on colonoscopy
C: 3.65 cm duodenal mass on endoscopy
B: Rectal mass on colonoscopy
C: 3.65 cm duodenal mass on endoscopy
Note: The table for this abstract can be viewed in the ePoster Gallery section of the ACG 2024 ePoster Site or in The American Journal of Gastroenterology's abstract supplement issue, both of which will be available starting October 27, 2024.
Disclosures:
Priyanka Shah indicated no relevant financial relationships.
Samyuktha Manikandan indicated no relevant financial relationships.
Manasa Vallabhaneni indicated no relevant financial relationships.
Frank Friedenberg indicated no relevant financial relationships.
Priyanka Shah, MD1, Samyuktha Manikandan, MD2, Manasa Vallabhaneni, MD2, Frank Friedenberg, MD, MS1. P4988 - Thinking Beyond the Colon: A Case of Duodenal Malignancy in a Patient With a Delayed Diagnosis of Lynch Syndrome, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
