Tuesday Poster Session
Category: Stomach
P5061 - Open Label Efficacy Analysis of Tradipitant in Idiopathic and Diabetic Gastroparesis
Tuesday, October 29, 2024
10:30 AM - 4:00 PM ET
Location: Exhibit Hall E
Has Audio

Jesse L. Carlin, PhD
Vanda Pharmaceuticals, Inc.
Washington, DC
Presenting Author(s)
Award: Presidential Poster Award
Jesse L.. Carlin, PhD, Christos Polymeropoulos, MD, Michaela Fisher, BS, Paula Moszczynski, MS, Garrett Johannsen, BS, Changfu Xiao, PhD, Gunther Birznieks, MS, Mihael Polymeropoulos, MD, PhD
Vanda Pharmaceuticals, Inc., Washington, DC
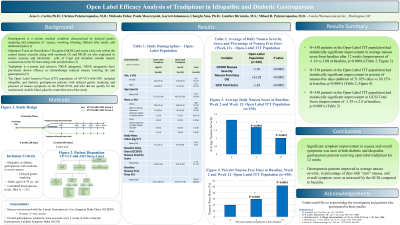
Introduction: Tradipitant is a novel NK1R antagonist studied in diabetic and idiopathic gastroparesis. This report presents the analysis from the open-label arm of a multicenter, randomized, double-blind, placebo-controlled study (VP-VLY-686-3301) assessing the efficacy of tradipitant in relieving symptoms of gastroparesis.
Methods: N=531 idiopathic and diabetic gastroparesis patients with delayed gastric emptying received open label 85mg tradipitant BID for 12 Weeks. Nausea was assessed using the 5-point Gastroparesis Core Symptom Daily Diary. An exposure response analysis was performed to control for low drug exposure. We used a threshold of ≤140ng/ml tradipitant blood concentration at any visit to classify participants in the High (n=309) and Low (n=218) PK Populations.
Results: Patients improved the average nausea score significantly from baseline after 12 weeks tradipitant (improvement of -1.01 vs 2.14 at baseline, p< 0.0001). A significant improvement was also seen in the percent of nausea free days (an addition of 51.76% of days for tradipitant vs. 19.62% at baseline, p< 0.0001).
Patients in the High PK group significantly improved average nausea score compared to the Low PK group at Week 12 (improvement of -1.19 vs -0.78, p< 0.0001). A significant improvement was also seen in the percent of nausea free days (an addition of 35.6% of days for High PK versus 22.6% for Low PK, p=0.0002).
Discussion: Tradipitant demonstrated statistically significant improvement from baseline in average nausea severity and percentage of nausea-free days in this 12 week open label study in both idiopathic and diabetic gastroparesis subjects. Additionally, a strong exposure response in nausea improvement was observed when comparing subjects with high drug exposure vs low drug exposure.
Disclosures:
Jesse L.. Carlin, PhD, Christos Polymeropoulos, MD, Michaela Fisher, BS, Paula Moszczynski, MS, Garrett Johannsen, BS, Changfu Xiao, PhD, Gunther Birznieks, MS, Mihael Polymeropoulos, MD, PhD. P5061 - Open Label Efficacy Analysis of Tradipitant in Idiopathic and Diabetic Gastroparesis, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
Jesse L.. Carlin, PhD, Christos Polymeropoulos, MD, Michaela Fisher, BS, Paula Moszczynski, MS, Garrett Johannsen, BS, Changfu Xiao, PhD, Gunther Birznieks, MS, Mihael Polymeropoulos, MD, PhD
Vanda Pharmaceuticals, Inc., Washington, DC
Introduction: Tradipitant is a novel NK1R antagonist studied in diabetic and idiopathic gastroparesis. This report presents the analysis from the open-label arm of a multicenter, randomized, double-blind, placebo-controlled study (VP-VLY-686-3301) assessing the efficacy of tradipitant in relieving symptoms of gastroparesis.
Methods: N=531 idiopathic and diabetic gastroparesis patients with delayed gastric emptying received open label 85mg tradipitant BID for 12 Weeks. Nausea was assessed using the 5-point Gastroparesis Core Symptom Daily Diary. An exposure response analysis was performed to control for low drug exposure. We used a threshold of ≤140ng/ml tradipitant blood concentration at any visit to classify participants in the High (n=309) and Low (n=218) PK Populations.
Results: Patients improved the average nausea score significantly from baseline after 12 weeks tradipitant (improvement of -1.01 vs 2.14 at baseline, p< 0.0001). A significant improvement was also seen in the percent of nausea free days (an addition of 51.76% of days for tradipitant vs. 19.62% at baseline, p< 0.0001).
Patients in the High PK group significantly improved average nausea score compared to the Low PK group at Week 12 (improvement of -1.19 vs -0.78, p< 0.0001). A significant improvement was also seen in the percent of nausea free days (an addition of 35.6% of days for High PK versus 22.6% for Low PK, p=0.0002).
Discussion: Tradipitant demonstrated statistically significant improvement from baseline in average nausea severity and percentage of nausea-free days in this 12 week open label study in both idiopathic and diabetic gastroparesis subjects. Additionally, a strong exposure response in nausea improvement was observed when comparing subjects with high drug exposure vs low drug exposure.
Disclosures:
Jesse Carlin: Vanda Pharmaceuticals, Inc. – Employee, Stock-publicly held company(excluding mutual/index funds).
Christos Polymeropoulos: Vanda Pharmaceuticals, Inc. – Employee, Stock-publicly held company(excluding mutual/index funds).
Michaela Fisher: Vanda Pharmaceuticals, Inc. – Employee, Stock-publicly held company(excluding mutual/index funds).
Paula Moszczynski: Vanda Pharmaceuticals, Inc. – Employee, Stock-publicly held company(excluding mutual/index funds).
Garrett Johannsen: Vanda Pharmaceuticals, Inc. – Employee, Stock-publicly held company(excluding mutual/index funds).
Changfu Xiao: Vanda Pharmaceuticals, Inc. – Employee, Stock-publicly held company(excluding mutual/index funds).
Gunther Birznieks: Vanda Pharmaceuticals, Inc. – Employee, Stock-publicly held company(excluding mutual/index funds).
Mihael Polymeropoulos: Vanda Pharmaceuticals, Inc. – Advisory Committee/Board Member, Employee, CEO, Stock-publicly held company(excluding mutual/index funds).
Jesse L.. Carlin, PhD, Christos Polymeropoulos, MD, Michaela Fisher, BS, Paula Moszczynski, MS, Garrett Johannsen, BS, Changfu Xiao, PhD, Gunther Birznieks, MS, Mihael Polymeropoulos, MD, PhD. P5061 - Open Label Efficacy Analysis of Tradipitant in Idiopathic and Diabetic Gastroparesis, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.

