Tuesday Poster Session
Category: Stomach
P5106 - Recurrent CMV Gastrointestinal Disease in an Immunosuppressed Patient With Solid Organ Transplant: Does Undetectable or Low Viral Load Matter?
Tuesday, October 29, 2024
10:30 AM - 4:00 PM ET
Location: Exhibit Hall E
Has Audio
- LN
Le Yu Naing, MD
University of Louisville
Louisville, KY
Presenting Author(s)
Le Yu Naing, MD1, Filsan Farah, MD1, Endashaw Omer, MD, MPH2
1University of Louisville, Louisville, KY; 2University of Louisville School of Medicine, Louisville, KY
Introduction: Recurrent cytomegalovirus (CMV) infection in immunosuppressed patients can be challenging to treat due to frequent reactivation from latent infection. An undetectable/low plasma/whole blood CMV quantitative PCR (qPCR) level does not exclude tissue-invasive disease. We present a case of recurrent CMV GI disease with low viral load in the presence of immunosuppression for kidney transplants.
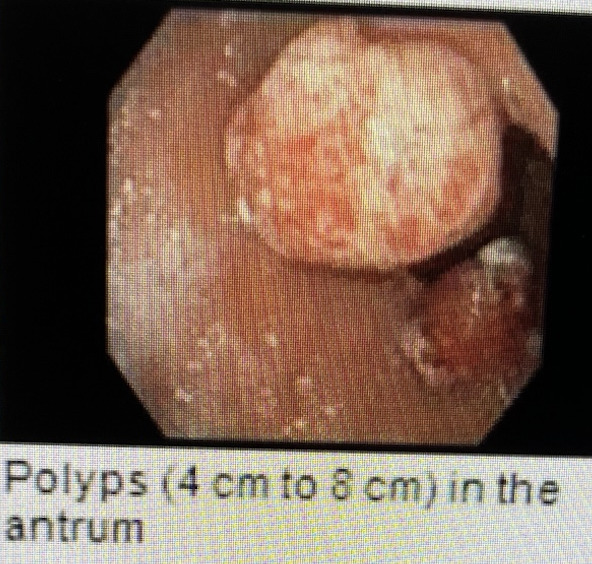
Case Description/Methods: The patient is a 63-year-old female with a history of renal transplant on immunosuppressants and a remote history of gastric adenocarcinoma, who presented with abdominal pain, nausea, vomiting, and unintentional weight loss of 30 lbs for 2 months. CMV viral load on admission was < 200 IU/ml (2.3 log IU/ml), reference range. She had a prior history of CMV gastritis and asymptomatic CMV viremia with a viral load of 1405 IU/ml. She responded to a two-week course of ganciclovir with clearance of viremia. EGD showed three 15-20 mm sessile gastric polyps with stigmata of recent bleeding in the prepyloric region of the stomach, and colonoscopy showed four polyps. Gastric polyp biopsy showed hyperplastic gastric polyps with acute inflammation and ulceration, and CMV immunostaining was positive in gastric and sigmoid colon polyps. She was treated with intravenous ganciclovir followed by oral therapy for four weeks. The anti-metabolite immunosuppressant was held initially and restarted with a reduced dose. She responded well without other tissue-invasive diseases.
Discussion: CMV in blood by nucleic acid amplification test or antigenemia alone is insufficient for diagnosing CMV GI disease, especially CMV R+ solid organ transplant recipients (IDSA consensus guideline).1 Compartmentalized CMV diseases- CMV retinitis or some cases of CMV GI diseases may not be detected by CMV qPCR in blood without systemic manifestations.1 Sensitivity of CMV qPCR is 85%, and specificity is 95%, both of which are lower with CMV seropositive recipients.2 The presence of CMV inclusions or positive CMV-specific immunohistochemistry staining is the gold standard for diagnosis.1 Viral culture of blood or urine is not recommended. First-line treatments for CMV disease are intravenous ganciclovir and oral valganciclovir until resolution of symptoms, virologic clearance below a threshold negative value, and a minimum of two weeks are achieved. Conclusion: Although CMV viral load helps guide treatment response, CMV infection in the GI tract cannot be excluded based on a negative plasma or whole blood PCR result.
Disclosures:
Le Yu Naing, MD1, Filsan Farah, MD1, Endashaw Omer, MD, MPH2. P5106 - Recurrent CMV Gastrointestinal Disease in an Immunosuppressed Patient With Solid Organ Transplant: Does Undetectable or Low Viral Load Matter?, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
1University of Louisville, Louisville, KY; 2University of Louisville School of Medicine, Louisville, KY
Introduction: Recurrent cytomegalovirus (CMV) infection in immunosuppressed patients can be challenging to treat due to frequent reactivation from latent infection. An undetectable/low plasma/whole blood CMV quantitative PCR (qPCR) level does not exclude tissue-invasive disease. We present a case of recurrent CMV GI disease with low viral load in the presence of immunosuppression for kidney transplants.
Case Description/Methods: The patient is a 63-year-old female with a history of renal transplant on immunosuppressants and a remote history of gastric adenocarcinoma, who presented with abdominal pain, nausea, vomiting, and unintentional weight loss of 30 lbs for 2 months. CMV viral load on admission was < 200 IU/ml (2.3 log IU/ml), reference range. She had a prior history of CMV gastritis and asymptomatic CMV viremia with a viral load of 1405 IU/ml. She responded to a two-week course of ganciclovir with clearance of viremia. EGD showed three 15-20 mm sessile gastric polyps with stigmata of recent bleeding in the prepyloric region of the stomach, and colonoscopy showed four polyps. Gastric polyp biopsy showed hyperplastic gastric polyps with acute inflammation and ulceration, and CMV immunostaining was positive in gastric and sigmoid colon polyps. She was treated with intravenous ganciclovir followed by oral therapy for four weeks. The anti-metabolite immunosuppressant was held initially and restarted with a reduced dose. She responded well without other tissue-invasive diseases.
Discussion: CMV in blood by nucleic acid amplification test or antigenemia alone is insufficient for diagnosing CMV GI disease, especially CMV R+ solid organ transplant recipients (IDSA consensus guideline).1 Compartmentalized CMV diseases- CMV retinitis or some cases of CMV GI diseases may not be detected by CMV qPCR in blood without systemic manifestations.1 Sensitivity of CMV qPCR is 85%, and specificity is 95%, both of which are lower with CMV seropositive recipients.2 The presence of CMV inclusions or positive CMV-specific immunohistochemistry staining is the gold standard for diagnosis.1 Viral culture of blood or urine is not recommended. First-line treatments for CMV disease are intravenous ganciclovir and oral valganciclovir until resolution of symptoms, virologic clearance below a threshold negative value, and a minimum of two weeks are achieved. Conclusion: Although CMV viral load helps guide treatment response, CMV infection in the GI tract cannot be excluded based on a negative plasma or whole blood PCR result.

Figure: Polyps (4cm to 8cm) in the antrum
Disclosures:
Le Yu Naing indicated no relevant financial relationships.
Filsan Farah indicated no relevant financial relationships.
Endashaw Omer indicated no relevant financial relationships.
Le Yu Naing, MD1, Filsan Farah, MD1, Endashaw Omer, MD, MPH2. P5106 - Recurrent CMV Gastrointestinal Disease in an Immunosuppressed Patient With Solid Organ Transplant: Does Undetectable or Low Viral Load Matter?, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
