Tuesday Poster Session
Category: Liver
P4825 - The Pesky Problem with Pembrolizumab: A Case of Chronic Hepatitis B Reactivation After Use of a Checkpoint Inhibitor
Tuesday, October 29, 2024
10:30 AM - 4:00 PM ET
Location: Exhibit Hall E
Has Audio
- CA
Casey Arnold, DO
Walter Reed National Military Medical Center
Portsmouth, VA
Presenting Author(s)
Casey Arnold, DO1, Kimberly Rivera, MD1, Shira Paul, MD1, Allison Bush, MD, MPH, LCDR, MC, USN2, Christie Joya, MD1, Michael Roth, MD1
1Walter Reed National Military Medical Center, Portsmouth, VA; 2Naval Medical Center Portsmouth, Portsmouth, VA
Introduction: Immune checkpoint inhibitor reactivation of Hepatitis B virus is described but not well understood. Reactivation is rare, occurring in 0.5% of HBVsAg positive patients, but is a risk that providers should be aware of (1). We present a case of Hepatitis B reactivation that occurred over a year and a half after treatment of esophageal cancer with pembrolizumab was completed.
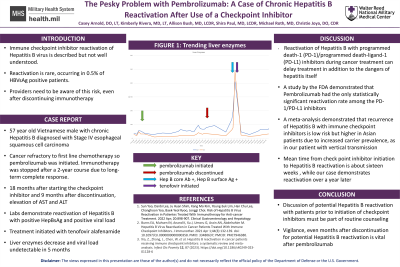
Case Description/Methods: A 57-year-old male of Vietnamese descent, with history of chronic hepatitis B with positive HBVcAb (presumed vertical transmission in neonatal period) was diagnosed with stage IV esophageal squamous cell carcinoma. Due to primary refractoriness to first-line chemotherapy, the patient was treated with palliative radiation to the esophageal mass, followed by a two-year course of pembrolizumab. As a result of a sustained complete response, pembrolizumab was discontinued after two years of treatment. Eighteen months later, unexpected elevation of liver enzymes (alanine transferase and aspartate aminotransferase greater than 400 U/L) were noted on routine esophageal cancer surveillance labs. Testing demonstrated positive HBVsAg and HBVeAb. Liver imaging demonstrated hepatic steatosis and labs resulted with thrombocytopenia suggesting some degree of fibrosis. He was started on treatment with tenofovir alafenamide with normalization of liver enzymes and undetectable viral load within five months.
Discussion: Reactivation of Hepatitis B with programmed death-1 (PD-1)/programmed death-ligand-1 (PD-L1) inhibitors during cancer treatment can cause delays in treatment compounded by the complications from Hepatitis B itself. A study by the FDA demonstrated that Pembrolizumab had the only statistical significance for the reactivation among other PD-1/PD-L1 inhibitors (2). A meta-analysis demonstrated that recurrence of Hepatitis B with immune checkpoint inhibitors is low risk but higher in Asian patients due to increased carrier prevalence (3). Our case is particularly interesting due to the timeline. Mean time from check point inhibitor initiation to Hepatitis B reactivation is about sixteen weeks (2). In our case, the reactivation occurred over a year later. Discussion with patients prior to initiation of this treatment and awareness of the possibility must become part of the routine counseling prior to pembrolizumab initiation.
Disclosures:
Casey Arnold, DO1, Kimberly Rivera, MD1, Shira Paul, MD1, Allison Bush, MD, MPH, LCDR, MC, USN2, Christie Joya, MD1, Michael Roth, MD1. P4825 - The Pesky Problem with Pembrolizumab: A Case of Chronic Hepatitis B Reactivation After Use of a Checkpoint Inhibitor, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
1Walter Reed National Military Medical Center, Portsmouth, VA; 2Naval Medical Center Portsmouth, Portsmouth, VA
Introduction: Immune checkpoint inhibitor reactivation of Hepatitis B virus is described but not well understood. Reactivation is rare, occurring in 0.5% of HBVsAg positive patients, but is a risk that providers should be aware of (1). We present a case of Hepatitis B reactivation that occurred over a year and a half after treatment of esophageal cancer with pembrolizumab was completed.
Case Description/Methods: A 57-year-old male of Vietnamese descent, with history of chronic hepatitis B with positive HBVcAb (presumed vertical transmission in neonatal period) was diagnosed with stage IV esophageal squamous cell carcinoma. Due to primary refractoriness to first-line chemotherapy, the patient was treated with palliative radiation to the esophageal mass, followed by a two-year course of pembrolizumab. As a result of a sustained complete response, pembrolizumab was discontinued after two years of treatment. Eighteen months later, unexpected elevation of liver enzymes (alanine transferase and aspartate aminotransferase greater than 400 U/L) were noted on routine esophageal cancer surveillance labs. Testing demonstrated positive HBVsAg and HBVeAb. Liver imaging demonstrated hepatic steatosis and labs resulted with thrombocytopenia suggesting some degree of fibrosis. He was started on treatment with tenofovir alafenamide with normalization of liver enzymes and undetectable viral load within five months.
Discussion: Reactivation of Hepatitis B with programmed death-1 (PD-1)/programmed death-ligand-1 (PD-L1) inhibitors during cancer treatment can cause delays in treatment compounded by the complications from Hepatitis B itself. A study by the FDA demonstrated that Pembrolizumab had the only statistical significance for the reactivation among other PD-1/PD-L1 inhibitors (2). A meta-analysis demonstrated that recurrence of Hepatitis B with immune checkpoint inhibitors is low risk but higher in Asian patients due to increased carrier prevalence (3). Our case is particularly interesting due to the timeline. Mean time from check point inhibitor initiation to Hepatitis B reactivation is about sixteen weeks (2). In our case, the reactivation occurred over a year later. Discussion with patients prior to initiation of this treatment and awareness of the possibility must become part of the routine counseling prior to pembrolizumab initiation.
Disclosures:
Casey Arnold indicated no relevant financial relationships.
Kimberly Rivera indicated no relevant financial relationships.
Shira Paul indicated no relevant financial relationships.
Allison Bush indicated no relevant financial relationships.
Christie Joya indicated no relevant financial relationships.
Michael Roth indicated no relevant financial relationships.
Casey Arnold, DO1, Kimberly Rivera, MD1, Shira Paul, MD1, Allison Bush, MD, MPH, LCDR, MC, USN2, Christie Joya, MD1, Michael Roth, MD1. P4825 - The Pesky Problem with Pembrolizumab: A Case of Chronic Hepatitis B Reactivation After Use of a Checkpoint Inhibitor, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
