Tuesday Poster Session
Category: IBD
P4437 - Ixekizumab-Induced Ulcerative Colitis With Resultant Total Colectomy
Tuesday, October 29, 2024
10:30 AM - 4:00 PM ET
Location: Exhibit Hall E
Has Audio
- CL
Connor Lovingood, MD
University of Tennessee Health Science Center
Chattanooga, TN
Presenting Author(s)
Award: Presidential Poster Award
Connor Lovingood, MD, James Laney, MD, William Oelsner, MD, Steven Kessler, DO, Sean Rice, MD, Arslan Kahloon, MD
University of Tennessee Health Science Center, Chattanooga, TN
Introduction: Ixekizumab is a human monoclonal antibody used to treat plaque psoriasis. It works by inhibiting interleukin-17A, (IL-17A) a cytokine involved in both psoriasis and inflammatory bowel disease (IBD). Counterintuitively, literature suggests ixekizumab is a possible inducer for IBD exacerbations. Clinical trials demonstrate a < 0.5% increase in frequency of exacerbations. Most previously cited exacerbations were mild and resolved with discontinuation of ixekizumab. There have been few documented cases of severe exacerbations refractory to standard medical therapy resulting in total colectomy.
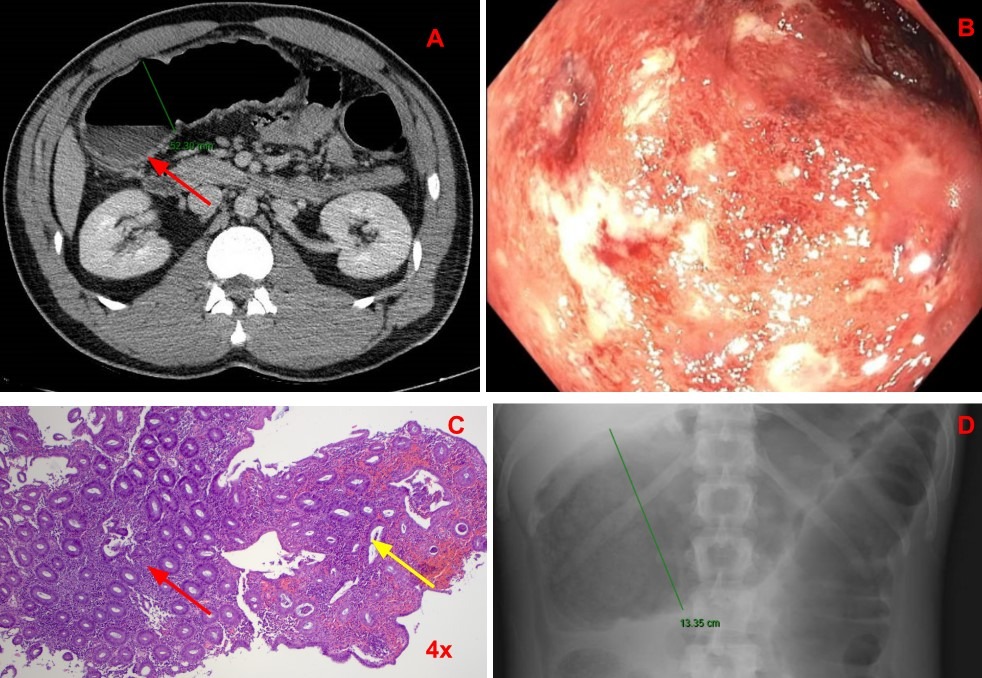
Case Description/Methods: A 36-year-old male started ixekizumab three months prior for plaque psoriasis presented for hematochezia and abdominal pain with several similar presentations to the emergency department over the previous two months. Initial presentations were mild and the patient was discharged home with follow-up. He was prescribed a course of amoxicillin and clavulanic acid as initial etiology was thought to be a combination of infectious colitis and internal hemorrhoids. Routine laboratory and stool testing were ordered and outpatient colonoscopy was scheduled, but never performed prior to the most recent presentation to the emergency department. He was found to have findings supportive of developing toxic megacolon on CT scan (Image 1A) and was deemed appropriate for medical management by surgical teams at that time. The patient underwent an unprepped colonoscopy, revealing inflamed, ulcerated mucosa of the rectum and sigmoid colon (Image 1B). Biopsy results showed diffuse active inflammation without evidence of granulomas or viral inclusion bodies, most suggestive of primary ulcerative colitis (Image 1C). Symptoms were refractory to high-dose methylprednisolone with worsening colonic dilatation (Image 1D). Salvage therapy was initiated with infliximab on day five without appropriate response, and he subsequently developed a colonic perforation at the splenic flexure at which point total colectomy was necessary.
Discussion: This case highlights the association between ixekizumab and induction/exacerbation of IBD. Although rare, our case and others demonstrate the necessity of screening patients for IBD prior to beginning treatment. Though the mechanisms are unknown, there is clear evidence that these patients should be monitored closely and treatment discontinued if concerns arise as our patient’s severe colitis unfortunately resulted in a total colectomy.
Disclosures:
Connor Lovingood, MD, James Laney, MD, William Oelsner, MD, Steven Kessler, DO, Sean Rice, MD, Arslan Kahloon, MD. P4437 - Ixekizumab-Induced Ulcerative Colitis With Resultant Total Colectomy, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
Connor Lovingood, MD, James Laney, MD, William Oelsner, MD, Steven Kessler, DO, Sean Rice, MD, Arslan Kahloon, MD
University of Tennessee Health Science Center, Chattanooga, TN
Introduction: Ixekizumab is a human monoclonal antibody used to treat plaque psoriasis. It works by inhibiting interleukin-17A, (IL-17A) a cytokine involved in both psoriasis and inflammatory bowel disease (IBD). Counterintuitively, literature suggests ixekizumab is a possible inducer for IBD exacerbations. Clinical trials demonstrate a < 0.5% increase in frequency of exacerbations. Most previously cited exacerbations were mild and resolved with discontinuation of ixekizumab. There have been few documented cases of severe exacerbations refractory to standard medical therapy resulting in total colectomy.
Case Description/Methods: A 36-year-old male started ixekizumab three months prior for plaque psoriasis presented for hematochezia and abdominal pain with several similar presentations to the emergency department over the previous two months. Initial presentations were mild and the patient was discharged home with follow-up. He was prescribed a course of amoxicillin and clavulanic acid as initial etiology was thought to be a combination of infectious colitis and internal hemorrhoids. Routine laboratory and stool testing were ordered and outpatient colonoscopy was scheduled, but never performed prior to the most recent presentation to the emergency department. He was found to have findings supportive of developing toxic megacolon on CT scan (Image 1A) and was deemed appropriate for medical management by surgical teams at that time. The patient underwent an unprepped colonoscopy, revealing inflamed, ulcerated mucosa of the rectum and sigmoid colon (Image 1B). Biopsy results showed diffuse active inflammation without evidence of granulomas or viral inclusion bodies, most suggestive of primary ulcerative colitis (Image 1C). Symptoms were refractory to high-dose methylprednisolone with worsening colonic dilatation (Image 1D). Salvage therapy was initiated with infliximab on day five without appropriate response, and he subsequently developed a colonic perforation at the splenic flexure at which point total colectomy was necessary.
Discussion: This case highlights the association between ixekizumab and induction/exacerbation of IBD. Although rare, our case and others demonstrate the necessity of screening patients for IBD prior to beginning treatment. Though the mechanisms are unknown, there is clear evidence that these patients should be monitored closely and treatment discontinued if concerns arise as our patient’s severe colitis unfortunately resulted in a total colectomy.

Figure: Image 1: Figure A CT scan showing new colonic distension of up to 5.23cm with colonic wall thickening (red arrow) and air-fluid levels concerning for early toxic megacolon. Figure B showing endoscopic visualization of diffuse colonic inflammation. Figure C a photomicrograph showing histologic evidence of active inflammation by increased number of plasma cells in lamina propria (red arrow) and chronic disease by architectural changes to crypts (yellow arrow). Figure D x-ray showing worsening colonic dilatation to 13.35cm.
Disclosures:
Connor Lovingood indicated no relevant financial relationships.
James Laney indicated no relevant financial relationships.
William Oelsner indicated no relevant financial relationships.
Steven Kessler indicated no relevant financial relationships.
Sean Rice indicated no relevant financial relationships.
Arslan Kahloon indicated no relevant financial relationships.
Connor Lovingood, MD, James Laney, MD, William Oelsner, MD, Steven Kessler, DO, Sean Rice, MD, Arslan Kahloon, MD. P4437 - Ixekizumab-Induced Ulcerative Colitis With Resultant Total Colectomy, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.

