Tuesday Poster Session
Category: IBD
P4335 - Efficacy of Etrasimod in Patients With Ulcerative Colitis: A Post Hoc Analysis Based on Baseline Endoscopic Subscore
Tuesday, October 29, 2024
10:30 AM - 4:00 PM ET
Location: Exhibit Hall E


Andres J. Yarur, MD
Associate Professor of Medicine
Inflammatory Bowel Disease Center, Cedars-Sinai Medical Center
Los Angeles, CA
Presenting Author(s)
Andres J. Yarur, MD1, Walter Reinisch, MD, PhD2, Shannon Chang, MD3, Krisztina B. Gecse, MD, PhD4, Jesse Green, MD5, Arcangelo M. Abbatemarco, MD6, Joseph Wu, PhD7, Martina Goetsch, MD8, Krisztina Lazin, MD8, Gokul Pradeep, 9, Bruce E.. Sands, MD, FACG10
1Inflammatory Bowel Disease Center, Cedars-Sinai Medical Center, Los Angeles, CA; 2Medical University of Vienna, Vienna, Wien, Austria; 3New York University Langone Health, New York, NY; 4Amsterdam University Medical Center, Amsterdam, Noord-Holland, Netherlands; 5Pfizer Inc., Collegeville, PA; 6Pfizer Inc., New York, NY; 7Pfizer Inc., Cambridge, MA; 8Pfizer AG, Zürich, Zurich, Switzerland; 9Pfizer Healthcare India Private Ltd., Chennai, Tamil Nadu, India; 10Icahn School of Medicine at Mount Sinai, New York, NY
Introduction: Severe colonic mucosal inflammation in patients with ulcerative colitis (UC) is associated with poor outcomes and increased risk of colectomy.1 Etrasimod is an oral, once-daily, selective sphingosine 1-phosphate (S1P)1,4,5 receptor modulator for the treatment of moderately to severely active UC. This post hoc analysis of ELEVATE UC 52 (NCT03945188) and ELEVATE UC 12 (NCT03996369) evaluated the efficacy of etrasimod vs placebo as induction and maintenance therapy based on baseline endoscopic severity.
Methods: At baseline, patients had a modified Mayo score of 4–9, a rectal bleeding subscore ≥ 1, and an endoscopic subscore (ES) ≥ 2. Endoscopic severity was graded by a blinded central reader, with an ES of 2 deemed moderate and an ES of 3 deemed severe disease. The primary endpoint (clinical remission) and secondary endpoints were assessed at the end of induction (Week [Wk] 12; pooled data) and at Wk 52.
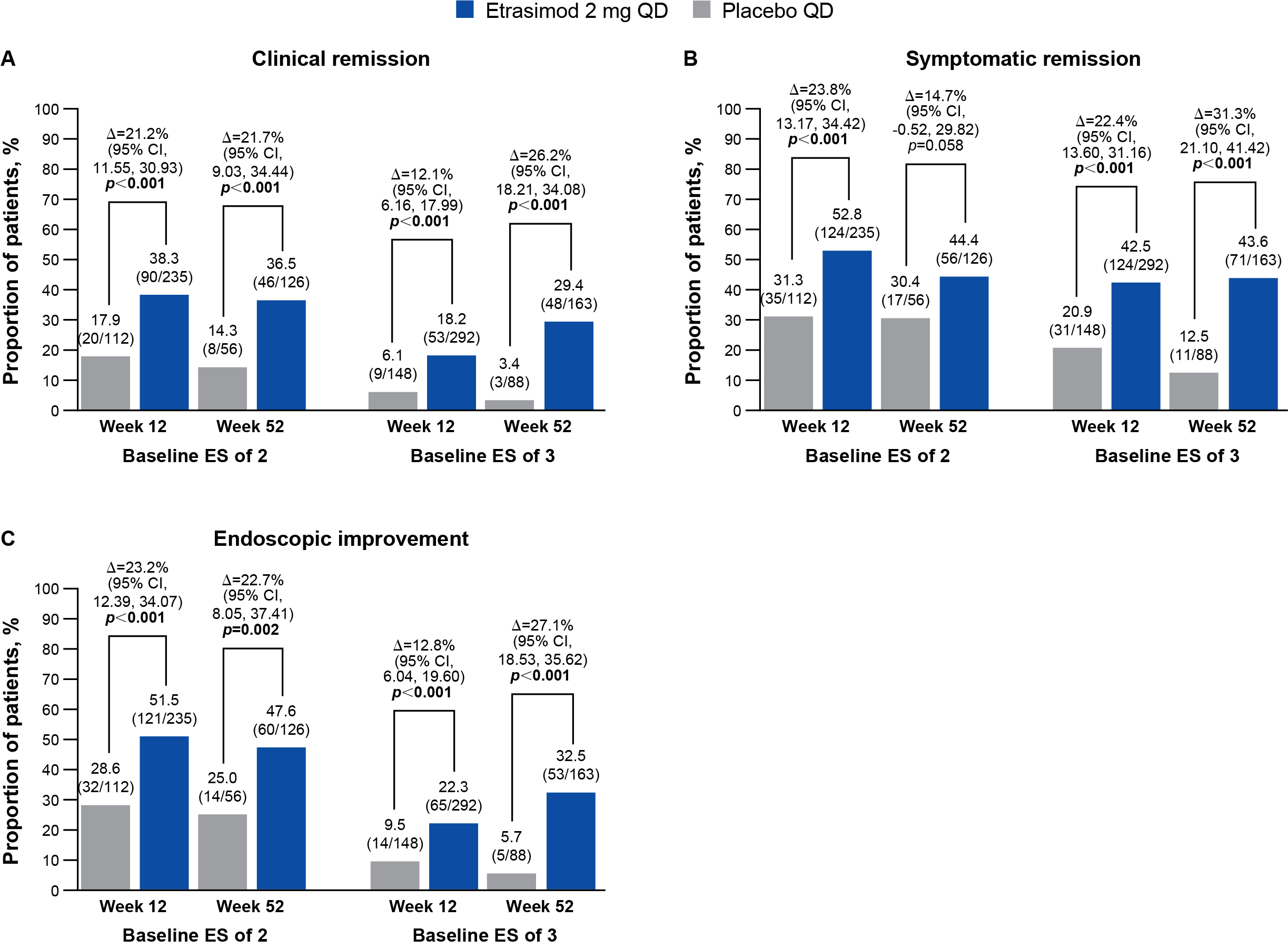
Results: In total, 235 patients with an ES of 2 and 292 patients with an ES of 3 received etrasimod; 112 and 148 patients, respectively, received placebo. At Wk 12, significantly greater proportions of patients receiving etrasimod vs placebo achieved clinical remission and secondary endpoints in the ES of 2 subgroup (p < 0.001), and all endpoints (p < 0.001) except endoscopic normalization (p = 0.083) in the ES of 3 subgroup (Figure; Table). At Wk 52, clinical remission (p < 0.001) and secondary endpoints (p < 0.05), except symptomatic remission (p = 0.058) and clinical response (p = 0.057), were achieved in the ES of 2 subgroup, and all endpoints in the ES of 3 subgroup (p < 0.001; Figure; Table). Adjusted difference in responses rates of etrasimod vs placebo for clinical remission, endoscopic improvement, and endoscopic normalization were generally similar between Wk 12 and Wk 52 in the ES of 2 subgroup, but higher at Wk 52 compared with Wk 12 in the ES of 3 subgroup (Figure; Table).
Discussion: Etrasimod demonstrated significant efficacy as induction and maintenance therapy over placebo in both endoscopic severity subgroups. Response to etrasimod in patients with severe endoscopic disease may further improve beyond 12-week induction therapy. This should be considered when assessing therapeutic response and setting treat-to-target strategies.
Pfizer’s generative artificial intelligence tool MAIA was used to assist production of the abstract first draft. Authors reviewed/edited and take responsibility for the content.
Reference:
1. Rubin DT et al. Gastroenterol Hepatol (NY) 2021;17:59–66.
Note: The table for this abstract can be viewed in the ePoster Gallery section of the ACG 2024 ePoster Site or in The American Journal of Gastroenterology's abstract supplement issue, both of which will be available starting October 27, 2024.
Disclosures:
Andres J. Yarur, MD1, Walter Reinisch, MD, PhD2, Shannon Chang, MD3, Krisztina B. Gecse, MD, PhD4, Jesse Green, MD5, Arcangelo M. Abbatemarco, MD6, Joseph Wu, PhD7, Martina Goetsch, MD8, Krisztina Lazin, MD8, Gokul Pradeep, 9, Bruce E.. Sands, MD, FACG10. P4335 - Efficacy of Etrasimod in Patients With Ulcerative Colitis: A Post Hoc Analysis Based on Baseline Endoscopic Subscore, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
1Inflammatory Bowel Disease Center, Cedars-Sinai Medical Center, Los Angeles, CA; 2Medical University of Vienna, Vienna, Wien, Austria; 3New York University Langone Health, New York, NY; 4Amsterdam University Medical Center, Amsterdam, Noord-Holland, Netherlands; 5Pfizer Inc., Collegeville, PA; 6Pfizer Inc., New York, NY; 7Pfizer Inc., Cambridge, MA; 8Pfizer AG, Zürich, Zurich, Switzerland; 9Pfizer Healthcare India Private Ltd., Chennai, Tamil Nadu, India; 10Icahn School of Medicine at Mount Sinai, New York, NY
Introduction: Severe colonic mucosal inflammation in patients with ulcerative colitis (UC) is associated with poor outcomes and increased risk of colectomy.1 Etrasimod is an oral, once-daily, selective sphingosine 1-phosphate (S1P)1,4,5 receptor modulator for the treatment of moderately to severely active UC. This post hoc analysis of ELEVATE UC 52 (NCT03945188) and ELEVATE UC 12 (NCT03996369) evaluated the efficacy of etrasimod vs placebo as induction and maintenance therapy based on baseline endoscopic severity.
Methods: At baseline, patients had a modified Mayo score of 4–9, a rectal bleeding subscore ≥ 1, and an endoscopic subscore (ES) ≥ 2. Endoscopic severity was graded by a blinded central reader, with an ES of 2 deemed moderate and an ES of 3 deemed severe disease. The primary endpoint (clinical remission) and secondary endpoints were assessed at the end of induction (Week [Wk] 12; pooled data) and at Wk 52.
Results: In total, 235 patients with an ES of 2 and 292 patients with an ES of 3 received etrasimod; 112 and 148 patients, respectively, received placebo. At Wk 12, significantly greater proportions of patients receiving etrasimod vs placebo achieved clinical remission and secondary endpoints in the ES of 2 subgroup (p < 0.001), and all endpoints (p < 0.001) except endoscopic normalization (p = 0.083) in the ES of 3 subgroup (Figure; Table). At Wk 52, clinical remission (p < 0.001) and secondary endpoints (p < 0.05), except symptomatic remission (p = 0.058) and clinical response (p = 0.057), were achieved in the ES of 2 subgroup, and all endpoints in the ES of 3 subgroup (p < 0.001; Figure; Table). Adjusted difference in responses rates of etrasimod vs placebo for clinical remission, endoscopic improvement, and endoscopic normalization were generally similar between Wk 12 and Wk 52 in the ES of 2 subgroup, but higher at Wk 52 compared with Wk 12 in the ES of 3 subgroup (Figure; Table).
Discussion: Etrasimod demonstrated significant efficacy as induction and maintenance therapy over placebo in both endoscopic severity subgroups. Response to etrasimod in patients with severe endoscopic disease may further improve beyond 12-week induction therapy. This should be considered when assessing therapeutic response and setting treat-to-target strategies.
Pfizer’s generative artificial intelligence tool MAIA was used to assist production of the abstract first draft. Authors reviewed/edited and take responsibility for the content.
Reference:
1. Rubin DT et al. Gastroenterol Hepatol (NY) 2021;17:59–66.

Figure: Figure. (A) Clinical remission[a], (B) symptomatic remission[b], and (C) endoscopic improvement[c] at Week 12 (pooled from ELEVATE UC 52 and ELEVATE UC 12) and Week 52 (ELEVATE UC 52 only), stratified by baseline ES subgroup (full analysis set).
Treatment comparisons (adjusted treatment difference and 95% CI) and 2-sided p values were obtained using the CMH method, adjusting to reported randomization stratification of prior biologic/JAKi therapy exposure, baseline CS use, baseline disease activity (MMS of 4–6 or 7–9), and study stratification (for pooled studies only). Missing response was considered as non-response. p<0.05 are highlighted in bold.
[a]Clinical remission was defined as an SFS = 0 (or = 1 with a ≥1-point decrease from baseline), RBS = 0 and
ES ≤1 (excluding friability).
[b]Symptomatic remission was defined as an SFS = 0 (or = 1 with a ≥1-point decrease from baseline) and an
RBS = 0.
[c]Endoscopic improvement was defined as an ES ≤1.
Abbreviations: Δ, adjusted percentage difference for etrasimod minus placebo; CI, confidence interval;
CMH, Cochran–Mantel–Haenszel; CS, corticosteroid; ES, endoscopic subscore; JAKi, Janus kinase inhibitor; MMS, modified Mayo score; QD, once daily; RBS, rectal bleeding subscore; SFS, stool frequency subscore; UC, ulcerative colitis.
Treatment comparisons (adjusted treatment difference and 95% CI) and 2-sided p values were obtained using the CMH method, adjusting to reported randomization stratification of prior biologic/JAKi therapy exposure, baseline CS use, baseline disease activity (MMS of 4–6 or 7–9), and study stratification (for pooled studies only). Missing response was considered as non-response. p<0.05 are highlighted in bold.
[a]Clinical remission was defined as an SFS = 0 (or = 1 with a ≥1-point decrease from baseline), RBS = 0 and
ES ≤1 (excluding friability).
[b]Symptomatic remission was defined as an SFS = 0 (or = 1 with a ≥1-point decrease from baseline) and an
RBS = 0.
[c]Endoscopic improvement was defined as an ES ≤1.
Abbreviations: Δ, adjusted percentage difference for etrasimod minus placebo; CI, confidence interval;
CMH, Cochran–Mantel–Haenszel; CS, corticosteroid; ES, endoscopic subscore; JAKi, Janus kinase inhibitor; MMS, modified Mayo score; QD, once daily; RBS, rectal bleeding subscore; SFS, stool frequency subscore; UC, ulcerative colitis.
Note: The table for this abstract can be viewed in the ePoster Gallery section of the ACG 2024 ePoster Site or in The American Journal of Gastroenterology's abstract supplement issue, both of which will be available starting October 27, 2024.
Disclosures:
Andres Yarur: AbbVie – Consultant, Lecture Fees. Arena – Consultant. Bristol Myers Squibb – Consultant, Lecture Fees. Pfizer Inc – Consultant. Takeda – Consultant.
Walter Reinisch: AbbVie – Advisory Committee/Board Member, Consultant, Grant/Research Support, Speakers Bureau. Amgen – Advisory Committee/Board Member, Consultant. AOP Orphan – Consultant. Boehringer Ingelheim – Advisory Committee/Board Member, Consultant. Bristol Myers Squibb – Advisory Committee/Board Member, Consultant. Calyx – Consultant. Celltrion – Advisory Committee/Board Member, Consultant, Speakers Bureau. Eli Lilly – Consultant. Ferring – Speakers Bureau. Galapagos – Advisory Committee/Board Member, Consultant, Speakers Bureau. Gilead – Consultant. Index Pharma – Consultant. Janssen – Advisory Committee/Board Member, Consultant, Grant/Research Support, Speakers Bureau. Medahead – Consultant. Microbiotica – Consultant. MSD – Speakers Bureau. Pfizer – Advisory Committee/Board Member, Consultant, Speakers Bureau. Roche – Speakers Bureau. Sandoz – Grant/Research Support. Sanofi – Grant/Research Support. Sobi – Speakers Bureau. Takeda – Consultant, Grant/Research Support, Speakers Bureau.
Shannon Chang: AbbVie – Consultant. Bristol Myers Squibb – Consultant. Janssen – Consultant. Pfizer – Consultant.
Krisztina Gecse: AbbVie – Consultant, Speakers Bureau. Celltrion – Consultant, Grant/Research Support, Speakers Bureau. Ferring Pharmaceuticals – Consultant, Speakers Bureau. Galapagos – Grant/Research Support. Immunic Therapeutics – Consultant, Speakers Bureau. Janssen – Consultant, Speakers Bureau. Novartis – Consultant, Speakers Bureau. Pfizer Inc – Consultant, Grant/Research Support, Speakers Bureau. Samsung Bioepis – Consultant, Speakers Bureau. Takeda – Consultant, Speakers Bureau. Tillots – Consultant, Speakers Bureau.
Jesse Green: Pfizer Inc. – Employee, Stock Options.
Arcangelo Abbatemarco: Pfizer Inc – Employee, Stock Options.
Joseph Wu: Pfizer Inc – Employee, Stock Options.
Martina Goetsch: Pfizer AG – Employee, Stock Options.
Krisztina Lazin: Pfizer AG – Employee. Pfizer Inc – Stock Options.
Gokul Pradeep: Pfizer Healthcare, India – Employee.
Bruce Sands: AbbVie – Consultant. Abivax – Consultant, Speakers Bureau. Adiso Therapeutics – Consultant. Agomab – Consultant. Alimentiv – Consultant. Amgen – Consultant. AnaptysBio – Consultant. Arena Pharmaceuticals – Consultant. Artugen Therapeutics – Consultant. AstraZeneca – Consultant. Biolojic Design – Consultant. Biora Therapeutics – Consultant. Boehringer Ingelheim – Consultant. Boston Pharmaceuticals – Consultant. Bristol Myers Squibb – Consultant, Grant/Research Support, Other support, Speakers Bureau. Calibr – Consultant. Celgene – Consultant. Celltrion – Consultant. ClostraBio – Consultant. Enthera – Consultant. Envied Biosciences – Consultant. Equilium – Consultant. Evommune – Consultant. Ferring – Consultant. Fiat – Consultant. Fresenius Kabi – Consultant. Galapagos – Consultant. Genentech (Roche) – Consultant. Gilead Sciences – Consultant. Glaxo SmithKline – Consultant. Gossamer Bio – Consultant. Imhotex – Consultant. Index Pharmaceuticals – Consultant. Innovation Pharmaceuticals – Consultant. Inotrem – Consultant. Janssen – Consultant, Grant/Research Support, Other support, Speakers Bureau. Kaleido – Consultant. Kallyope – Consultant. Lilly – Consultant, other support, Speakers Bureau. Merck & Co., Inc., Rahway, NJ, USA – Consultant. Microba – Consultant. Mobius Care – Consultant. Morphic Therapeutics – Consultant. MRM Health – Consultant. Nexus Therapeutics – Consultant. Nimbus Discovery – Consultant. Odyssey Therapeutics – Consultant. Pfizer Inc – Consultant, Grant/Research Support, Other support, Speakers Bureau. Progenity – Consultant. Prometheus Biosciences – Consultant. Prometheus Laboratories – Consultant. Protagonist Therapeutics – Consultant. Q32 Bio – Consultant. Rasayana Therapeutics – Consultant. Recludix Therapeutics – Consultant. Reistone Biopharma – Consultant. Sanofi – Consultant. Spyre Therapeutics – Consultant. Sun Pharma – Consultant. Surrozen – Consultant. Takeda – Consultant, Grant/Research Support, Other support, Speakers Bureau. Target RWE – Consultant. Teva – Consultant. Theravance Biopharma – Consultant, Grant/Research Support. TLL Pharmaceutical – Consultant. Tr1X – Consultant. Union Therapeutics – Consultant. Ventyx Biopharma – Consultant, Stock Options, Stock-publicly held company(excluding mutual/index funds).
Andres J. Yarur, MD1, Walter Reinisch, MD, PhD2, Shannon Chang, MD3, Krisztina B. Gecse, MD, PhD4, Jesse Green, MD5, Arcangelo M. Abbatemarco, MD6, Joseph Wu, PhD7, Martina Goetsch, MD8, Krisztina Lazin, MD8, Gokul Pradeep, 9, Bruce E.. Sands, MD, FACG10. P4335 - Efficacy of Etrasimod in Patients With Ulcerative Colitis: A Post Hoc Analysis Based on Baseline Endoscopic Subscore, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
