Tuesday Poster Session
Category: IBD
P4336 - Efficacy and Safety of Etrasimod in Patients With Ulcerative Colitis Stratified by Body Mass Index: A Post Hoc Analysis From the ELEVATE UC Clinical Program
Tuesday, October 29, 2024
10:30 AM - 4:00 PM ET
Location: Exhibit Hall E

Andres J. Yarur, MD
Associate Professor of Medicine
Inflammatory Bowel Disease Center, Cedars-Sinai Medical Center
Los Angeles, CA
Presenting Author(s)
Andres J. Yarur, MD1, Millie D. Long, MD, MPH2, Joana Torres, MD, PhD3, Neilanjan Nandi, MD4, Raymond K. Cross, MD, MS, FACG5, Arcangelo M. Abbatemarco, MD6, David Blanco, 7, Wojciech Niezychowski, MD7, Catherine M. Crosby, PhD8, Joseph Wu, PhD9, Gokul Pradeep, 10, Martina Goetsch, MD11, Remo Panaccione, MD12
1Inflammatory Bowel Disease Center, Cedars-Sinai Medical Center, Los Angeles, CA; 2Center for Gastrointestinal Biology and Disease, University of North Carolina, Chapel Hill, NC; 3Hospital da Luz, Hospital Beatriz Angelo, Faculdade de Medicina, Universidade de Lisboa, Lisbon, Lisboa, Portugal; 4Penn Presbyterian Medical Center, University of Pennsylvania, Philadelphia, PA; 5Melissa L. Posner Institute for Digestive Health & Liver Disease at Mercy Medical Center, Baltimore, MD; 6Pfizer Inc., New York, NY; 7Pfizer Inc., Collegeville, PA; 8Pfizer Inc., San Diego, CA; 9Pfizer Inc., Cambridge, MA; 10Pfizer Healthcare India Private Ltd., Chennai, Tamil Nadu, India; 11Pfizer AG, Zürich, Zurich, Switzerland; 12University of Calgary, Calgary, AB, Canada
Introduction: High body mass index (BMI) may negatively impact treatment efficacy of advanced therapies, like biologics, in patients (pts) with ulcerative colitis (UC).1 The prevalence of obesity and UC have increased substantially in recent decades.2,3 Etrasimod is an oral, once-daily (QD), selective sphingosine 1‑phosphate (S1P)1,4,5 receptor modulator for the treatment of moderately to severely active UC.
Methods: This post hoc analysis of ELEVATE UC 52 and ELEVATE UC 12 investigated the impact of BMI on the efficacy and safety of etrasimod in pts with UC. ELEVATE UC 52 (NCT03945188; treat-through design with 12-week [wk] induction and 40-wk maintenance periods) and ELEVATE UC 12 (NCT03996369; 12-wk induction period) were phase 3, randomized, placebo (PBO)-controlled trials.4 Pts taking ≥ 1 dose of etrasimod 2 mg QD or PBO were stratified by baseline BMI: < 25 kg/m2, 25–30 kg/m2, and > 30 kg/m2. Efficacy outcomes (per predefined outcomes) included clinical remission (primary endpoint) and selected secondary endpoints at Wk 12 (pooled) and Wk 52 (ELEVATE UC 52). Safety outcomes assessed treatment‑emergent adverse event (TEAE) proportions.
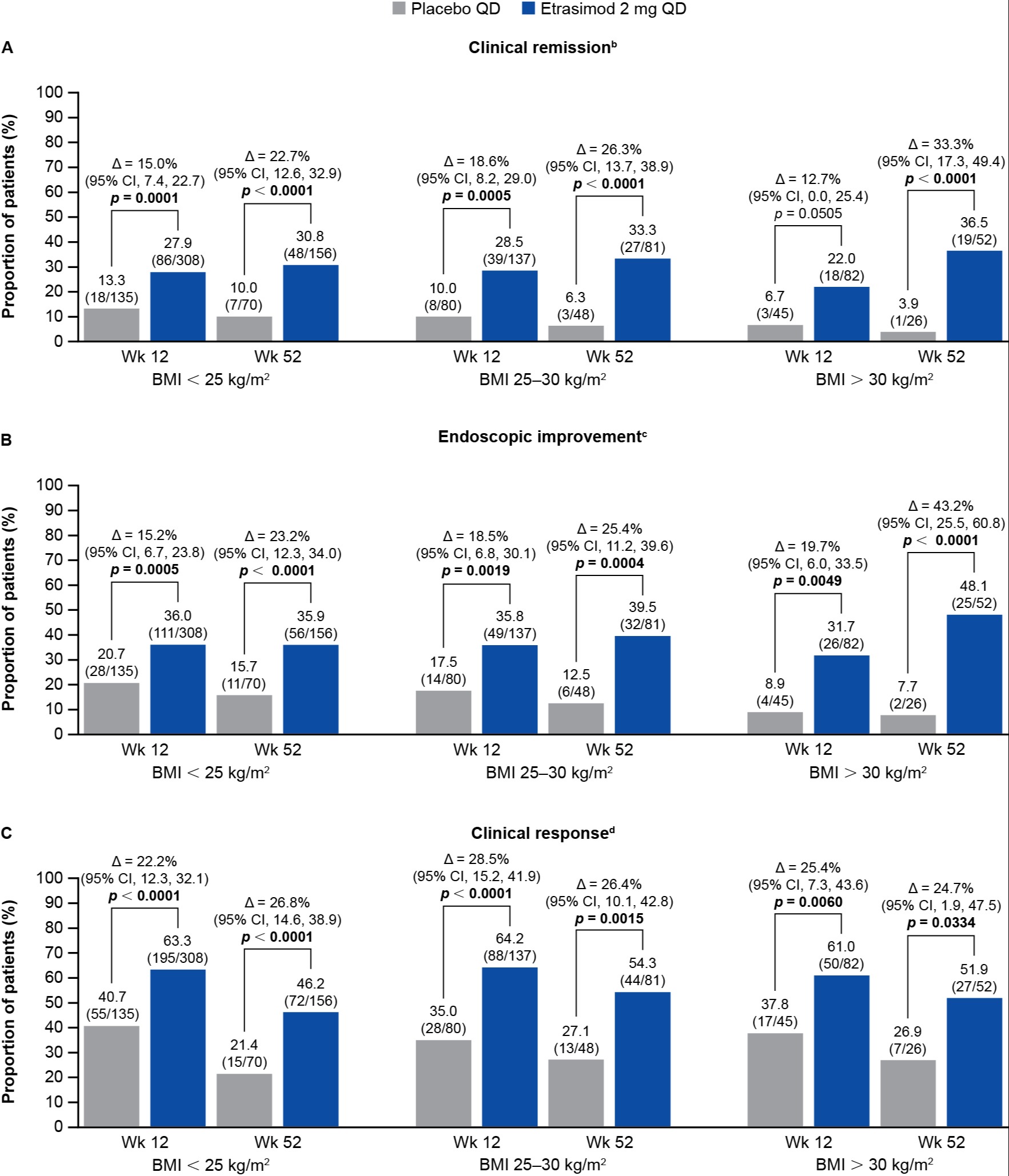
Results: Of the 787 pts in the ELEVATE UC clinical program, 443 (56.3%), 217 (27.6%), and 127 (16.1%) had baseline BMI < 25 kg/m2, 25–30 kg/m2, and > 30 kg/m2, respectively. Significantly more pts with BMI < 25 kg/m2 and 25–30 kg/m2 taking etrasimod vs PBO achieved all efficacy endpoints at Wks 12 and 52 (p < 0.05; Figure). Significantly more pts with BMI > 30 kg/m2 taking etrasimod vs PBO achieved clinical remission at Wk 52 (p < 0.0001; Wk 12: p = 0.05), endoscopic improvement and clinical response at Wks 12 and 52 (Figure), and corticosteroid‑free clinical remission at Wk 52 (all p < 0.05). The proportions of pts with the most common TEAEs were similar between BMI subgroups taking etrasimod (Table). Serious AE proportions were generally similar across BMI subgroups taking etrasimod (< 25 kg/m2: 17/308 [5.5%]; 25–30 kg/m2: 8/137 [5.8%]; > 30 kg/m2: 1/82 [1.2%]).
Discussion: In the ELEVATE UC program, there were significant improvements in efficacy with etrasimod 2 mg QD vs PBO, regardless of baseline BMI. The safety profile of etrasimod was consistent between BMI subgroups and with the overall ELEVATE UC population.
References:
1. Kurnool S et al. Aliment Pharmacol Ther 2018; 47: 1472–1479.
2. Molodecky NA et al. Gastroenterology 2012; 142: 46–54.e42; quiz e30.
3. Ng M et al. Lancet 2014; 384: 766–781.
4. Sandborn WJ et al. J Crohns Colitis 2023; 17: 338–351.
Note: The table for this abstract can be viewed in the ePoster Gallery section of the ACG 2024 ePoster Site or in The American Journal of Gastroenterology's abstract supplement issue, both of which will be available starting October 27, 2024.
Disclosures:
Andres J. Yarur, MD1, Millie D. Long, MD, MPH2, Joana Torres, MD, PhD3, Neilanjan Nandi, MD4, Raymond K. Cross, MD, MS, FACG5, Arcangelo M. Abbatemarco, MD6, David Blanco, 7, Wojciech Niezychowski, MD7, Catherine M. Crosby, PhD8, Joseph Wu, PhD9, Gokul Pradeep, 10, Martina Goetsch, MD11, Remo Panaccione, MD12. P4336 - Efficacy and Safety of Etrasimod in Patients With Ulcerative Colitis Stratified by Body Mass Index: A Post Hoc Analysis From the ELEVATE UC Clinical Program, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
1Inflammatory Bowel Disease Center, Cedars-Sinai Medical Center, Los Angeles, CA; 2Center for Gastrointestinal Biology and Disease, University of North Carolina, Chapel Hill, NC; 3Hospital da Luz, Hospital Beatriz Angelo, Faculdade de Medicina, Universidade de Lisboa, Lisbon, Lisboa, Portugal; 4Penn Presbyterian Medical Center, University of Pennsylvania, Philadelphia, PA; 5Melissa L. Posner Institute for Digestive Health & Liver Disease at Mercy Medical Center, Baltimore, MD; 6Pfizer Inc., New York, NY; 7Pfizer Inc., Collegeville, PA; 8Pfizer Inc., San Diego, CA; 9Pfizer Inc., Cambridge, MA; 10Pfizer Healthcare India Private Ltd., Chennai, Tamil Nadu, India; 11Pfizer AG, Zürich, Zurich, Switzerland; 12University of Calgary, Calgary, AB, Canada
Introduction: High body mass index (BMI) may negatively impact treatment efficacy of advanced therapies, like biologics, in patients (pts) with ulcerative colitis (UC).1 The prevalence of obesity and UC have increased substantially in recent decades.2,3 Etrasimod is an oral, once-daily (QD), selective sphingosine 1‑phosphate (S1P)1,4,5 receptor modulator for the treatment of moderately to severely active UC.
Methods: This post hoc analysis of ELEVATE UC 52 and ELEVATE UC 12 investigated the impact of BMI on the efficacy and safety of etrasimod in pts with UC. ELEVATE UC 52 (NCT03945188; treat-through design with 12-week [wk] induction and 40-wk maintenance periods) and ELEVATE UC 12 (NCT03996369; 12-wk induction period) were phase 3, randomized, placebo (PBO)-controlled trials.4 Pts taking ≥ 1 dose of etrasimod 2 mg QD or PBO were stratified by baseline BMI: < 25 kg/m2, 25–30 kg/m2, and > 30 kg/m2. Efficacy outcomes (per predefined outcomes) included clinical remission (primary endpoint) and selected secondary endpoints at Wk 12 (pooled) and Wk 52 (ELEVATE UC 52). Safety outcomes assessed treatment‑emergent adverse event (TEAE) proportions.
Results: Of the 787 pts in the ELEVATE UC clinical program, 443 (56.3%), 217 (27.6%), and 127 (16.1%) had baseline BMI < 25 kg/m2, 25–30 kg/m2, and > 30 kg/m2, respectively. Significantly more pts with BMI < 25 kg/m2 and 25–30 kg/m2 taking etrasimod vs PBO achieved all efficacy endpoints at Wks 12 and 52 (p < 0.05; Figure). Significantly more pts with BMI > 30 kg/m2 taking etrasimod vs PBO achieved clinical remission at Wk 52 (p < 0.0001; Wk 12: p = 0.05), endoscopic improvement and clinical response at Wks 12 and 52 (Figure), and corticosteroid‑free clinical remission at Wk 52 (all p < 0.05). The proportions of pts with the most common TEAEs were similar between BMI subgroups taking etrasimod (Table). Serious AE proportions were generally similar across BMI subgroups taking etrasimod (< 25 kg/m2: 17/308 [5.5%]; 25–30 kg/m2: 8/137 [5.8%]; > 30 kg/m2: 1/82 [1.2%]).
Discussion: In the ELEVATE UC program, there were significant improvements in efficacy with etrasimod 2 mg QD vs PBO, regardless of baseline BMI. The safety profile of etrasimod was consistent between BMI subgroups and with the overall ELEVATE UC population.
References:
1. Kurnool S et al. Aliment Pharmacol Ther 2018; 47: 1472–1479.
2. Molodecky NA et al. Gastroenterology 2012; 142: 46–54.e42; quiz e30.
3. Ng M et al. Lancet 2014; 384: 766–781.
4. Sandborn WJ et al. J Crohns Colitis 2023; 17: 338–351.

Figure: Figure. Primary and selected secondary efficacy endpoints at Wk 12 (pooled data from ELEVATE UC 52 and ELEVATE UC 12) and at Wk 52 (ELEVATE UC 52),[a] stratified by baseline BMI.
Data labels present percentage value with n/N in brackets underneath.
Differences (95% CI) and 2-sided p values are based on the Cochran–Mantel–Haenszel method adjusting to reported randomization stratification of (1) naïve to biologic or Janus kinase inhibitor therapy at study entry, (2) baseline CS use (yes or no), (3) baseline disease activity (MMS: 4–6 or 7–9), and (4), for pooled Wk 12 data only, study identifier (ELEVATE UC 52 and ELEVATE UC 12).
[a]Data were pooled at Wk 12 for both trials (ELEVATE UC 52 and ELEVATE UC 12); Wk 52 is ELEVATE UC 52 only.
[b]Clinical remission was defined as SFS = 0 (or = 1 with a ≥ 1-point decrease from baseline), RBS = 0, and ES ≤ 1 (excluding friability).
[c]Endoscopic improvement was defined as ES ≤ 1.
[d]Clinical response was defined as a ≥ 2-point and ≥ 30% decrease from baseline in MMS, and a ≥ 1-point decrease from baseline in RBS or an absolute RBS ≤ 1.
∆, difference; BMI, body mass index; CI, confidence interval; CS, corticosteroid; ES, endoscopic subscore; MMS, modified Mayo score; n, number of patients; N, number of patients in the BMI subgroup; QD, once daily; RBS, rectal bleeding subscore; SFS, stool frequency subscore; UC, ulcerative colitis; Wk, Week.
Data labels present percentage value with n/N in brackets underneath.
Differences (95% CI) and 2-sided p values are based on the Cochran–Mantel–Haenszel method adjusting to reported randomization stratification of (1) naïve to biologic or Janus kinase inhibitor therapy at study entry, (2) baseline CS use (yes or no), (3) baseline disease activity (MMS: 4–6 or 7–9), and (4), for pooled Wk 12 data only, study identifier (ELEVATE UC 52 and ELEVATE UC 12).
[a]Data were pooled at Wk 12 for both trials (ELEVATE UC 52 and ELEVATE UC 12); Wk 52 is ELEVATE UC 52 only.
[b]Clinical remission was defined as SFS = 0 (or = 1 with a ≥ 1-point decrease from baseline), RBS = 0, and ES ≤ 1 (excluding friability).
[c]Endoscopic improvement was defined as ES ≤ 1.
[d]Clinical response was defined as a ≥ 2-point and ≥ 30% decrease from baseline in MMS, and a ≥ 1-point decrease from baseline in RBS or an absolute RBS ≤ 1.
∆, difference; BMI, body mass index; CI, confidence interval; CS, corticosteroid; ES, endoscopic subscore; MMS, modified Mayo score; n, number of patients; N, number of patients in the BMI subgroup; QD, once daily; RBS, rectal bleeding subscore; SFS, stool frequency subscore; UC, ulcerative colitis; Wk, Week.
Note: The table for this abstract can be viewed in the ePoster Gallery section of the ACG 2024 ePoster Site or in The American Journal of Gastroenterology's abstract supplement issue, both of which will be available starting October 27, 2024.
Disclosures:
Andres Yarur: AbbVie – Consultant, Lecture Fees. Arena – Consultant. Bristol Myers Squibb – Consultant, Lecture Fees. Pfizer Inc – Consultant. Takeda – Consultant.
Millie Long: AbbVie – Consultant. Bristol-Myers Squibb – Consultant. Eli Lilly – Consultant, Grant/Research Support. Intercept – Consultant. Janssen – Consultant. Pfizer Inc – Consultant, Grant/Research Support. Prometheus – Consultant. Roche – Consultant. Roivant – Consultant. Takeda – Consultant, Grant/Research Support. Target RWE – Consultant.
Joana Torres: AbbVie – Advisory Committee/Board Member, Grant/Research Support, Speakers Bureau. Arena – Advisory Committee/Board Member, Speaker Fees. Bristol Myers Squibb – Advisory Committee/Board Member, Speakers Bureau. Janssen – Advisory Committee/Board Member, Grant/Research Support, Speakers Bureau. Pfizer – Advisory Committee/Board Member, Speakers Bureau. Sandoz – Advisory Committee/Board Member, Speakers Bureau.
Neilanjan Nandi: AbbVie – Advisory Committee/Board Member, Consultant. Bristol Myers Squibb – Advisory Committee/Board Member, Consultant. Eli Lilly – Advisory Committee/Board Member, Consultant. Ferring – Grant/Research Support. Janssen – Advisory Committee/Board Member, Consultant, Grant/Research Support. Pfizer Inc – Advisory Committee/Board Member, Consultant, Grant/Research Support. Seres – Grant/Research Support.
Raymond Cross: AbbVie – Consultant. Adiso – Consultant. Bristol Myers Squibb – Consultant. CorEvitas Registry – Scientific co-director. Fresenius Kabi – Consultant. Fzata – Consultant. IBD Education Group – Executive committee member. Janssen – Consultant, Grant/Research Support. Magellan Health – Consultant. Option Care Health – Consultant. Pfizer – Consultant. Pharmacosmos – Consultant. Samsung Bioepis – Consultant. Sandoz – Consultant. Sebela – Consultant. Takeda – Consultant.
Arcangelo Abbatemarco: Pfizer Inc – Employee, Stock Options.
David Blanco: Pfizer Inc – Was an employee of Pfizer Inc at the time of the analysis, Stock Options.
Wojciech Niezychowski: Pfizer Inc – Employee.
Catherine Crosby: Pfizer Inc – Employee, Stock Options.
Joseph Wu: Pfizer Inc – Employee, Stock Options.
Gokul Pradeep: Pfizer Healthcare, India – Employee.
Martina Goetsch: Pfizer AG – Employee, Stock Options.
Remo Panaccione: Élan – Consultant. Abbivax – Consultant. Abbott – Consultant. AbbVie – Advisory Committee/Board Member, Consultant, Speaking Fees. Alimentiv – Advisory Committee/Board Member, Consultant. Amgen – Advisory Committee/Board Member, Consultant, Speaker Fees. Arena Pharmaceuticals – Advisory Committee/Board Member, Consultant, Speaker Fees. AstraZeneca – Advisory Committee/Board Member, Consultant. Biogen – Advisory Committee/Board Member, Consultant. Boehringer Ingelheim – Advisory Committee/Board Member, Consultant. Bristol Myers Squibb – Advisory Committee/Board Member, Consultant, Speaker Fees. Celgene – Advisory Committee/Board Member, Consultant, Speaker Fees. Celltrion – Consultant. Cosmos Pharmaceuticals – Consultant. Eisai – Consultant. Ferring – Advisory Committee/Board Member, Consultant, Speaker Fees. Fresenius Kabi – Advisory Committee/Board Member, Consultant, Speaker Fees. Galapagos – Consultant. Genentech – Advisory Committee/Board Member, Consultant. Gilead – Advisory Committee/Board Member, Consultant, Speaker Fees. GlaxoSmithKline – Advisory Committee/Board Member, Consultant. JAMP Bio – Advisory Committee/Board Member, Consultant. Janssen – Advisory Committee/Board Member, Consultant, Speaker Fees. Lilly – Advisory Committee/Board Member, Consultant, Speaker Fees. Merck – Advisory Committee/Board Member, Consultant, Speaker Fees. Mylan – Advisory Committee/Board Member, Consultant. Novartis – Advisory Committee/Board Member, Consultant. Oppilan Pharma – Advisory Committee/Board Member, Consultant. Organon – Advisory Committee/Board Member, Consultant, Speaker Fees. Pandion Pharma – Advisory Committee/Board Member, Consultant. Pendopharm – Consultant. Pfizer Inc – Advisory Committee/Board Member, Consultant, Speaker Fees. Progenity – Advisory Committee/Board Member, Consultant. Prometheus Biosciences – Consultant. Protagonist Therapeutics – Advisory Committee/Board Member, Consultant. Roche – Advisory Committee/Board Member, Consultant, Speaker Fees. Sandoz – Advisory Committee/Board Member, Consultant, Speaker Fees. Satisfai Health – Consultant. Shire – Advisory Committee/Board Member, Consultant, Speaker Fees. Sublimity Therapeutics – Advisory Committee/Board Member, Consultant. Takeda – Advisory Committee/Board Member, Consultant, Speaker Fees. Theravance – Consultant. Trellus – Consultant. UCB – Consultant. Ventyx – Advisory Committee/Board Member, Consultant. Viatris – Consultant.
Andres J. Yarur, MD1, Millie D. Long, MD, MPH2, Joana Torres, MD, PhD3, Neilanjan Nandi, MD4, Raymond K. Cross, MD, MS, FACG5, Arcangelo M. Abbatemarco, MD6, David Blanco, 7, Wojciech Niezychowski, MD7, Catherine M. Crosby, PhD8, Joseph Wu, PhD9, Gokul Pradeep, 10, Martina Goetsch, MD11, Remo Panaccione, MD12. P4336 - Efficacy and Safety of Etrasimod in Patients With Ulcerative Colitis Stratified by Body Mass Index: A Post Hoc Analysis From the ELEVATE UC Clinical Program, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
