Tuesday Poster Session
Category: IBD
P4339 - Efficacy and Safety of Etrolizumab in the Treatment of Moderate to Severe Ulcerative Colitis: A Systematic Review and Meta-Analysis of Randomized Controlled Trials
Tuesday, October 29, 2024
10:30 AM - 4:00 PM ET
Location: Exhibit Hall E

- FJ
Fouad Jaber, MD, MS
University of Missouri - Kansas City School of Medicine
Kansas City, MO
Presenting Author(s)
Fouad Jaber, MD, MS1, Saqr Alsakarneh, MD1, Mohammed Ayyad, MD2, Tala Alsharaeh, MD3, Ahmed-Jordan Salahat, MD3, Mohammad Jaber, MD4, Yassine Kilani, MD5, Mohammad Aldiabat, MD6, Manesh Kumar Gangwani, MD7, Yazan Abboud, MD2, Ahmed Fares, MD8, Dushyant S. Dahiya, MD9, Mohamed Ahmed, MD10, Yazan Sallam, MD11, Richa Shukla, MD12
1University of Missouri - Kansas City School of Medicine, Kansas City, MO; 2Rutgers New Jersey Medical School, Newark, NJ; 3The University of Jordan, Amman, 'Amman, Jordan; 4Al-Azhar University, Gaza, Palestinian Territories; 5SSM Health Saint Louis University Hospital, St. Louis, MO; 6Washington University in St. Louis, St. Louis, MO; 7University of Toledo, Toledo, OH; 8Tufts Medical Center, Boston, MA; 9The University of Kansas School of Medicine, Kansas City, KS; 10University of Missouri-Kansas City, Saint Luke's Hospital, Kansas City, MO; 11University of Missouri, Kansas City, MO; 12Baylor College of Medicine, Houston, TX
Introduction: Etrolizumab is not yet commercially available for UC treatment. Despite the promising outcomes and safety profile of etrolizumab owing to its selective mechanism of action, the certainty behind its efficacy in the treatment of moderate to severe UC remains unclear. Our study aims to evaluate the effectiveness and safety of etrolizumab, a monoclonal antibody targeting integrin molecules, particularly the β7 subunit in α4β7 and αEβ7 integrin heterodimers, for the treatment of moderate to severe ulcerative colitis (UC).
Methods: We conducted a systematic review and meta-analysis, gathering randomized controlled trials (RCTs) from PubMed, Web of Science, SCOPUS, and Cochrane up to January 7th, 2023. Using a fixed-effect model, we pooled dichotomous data with RR and 95% CI for analysis.
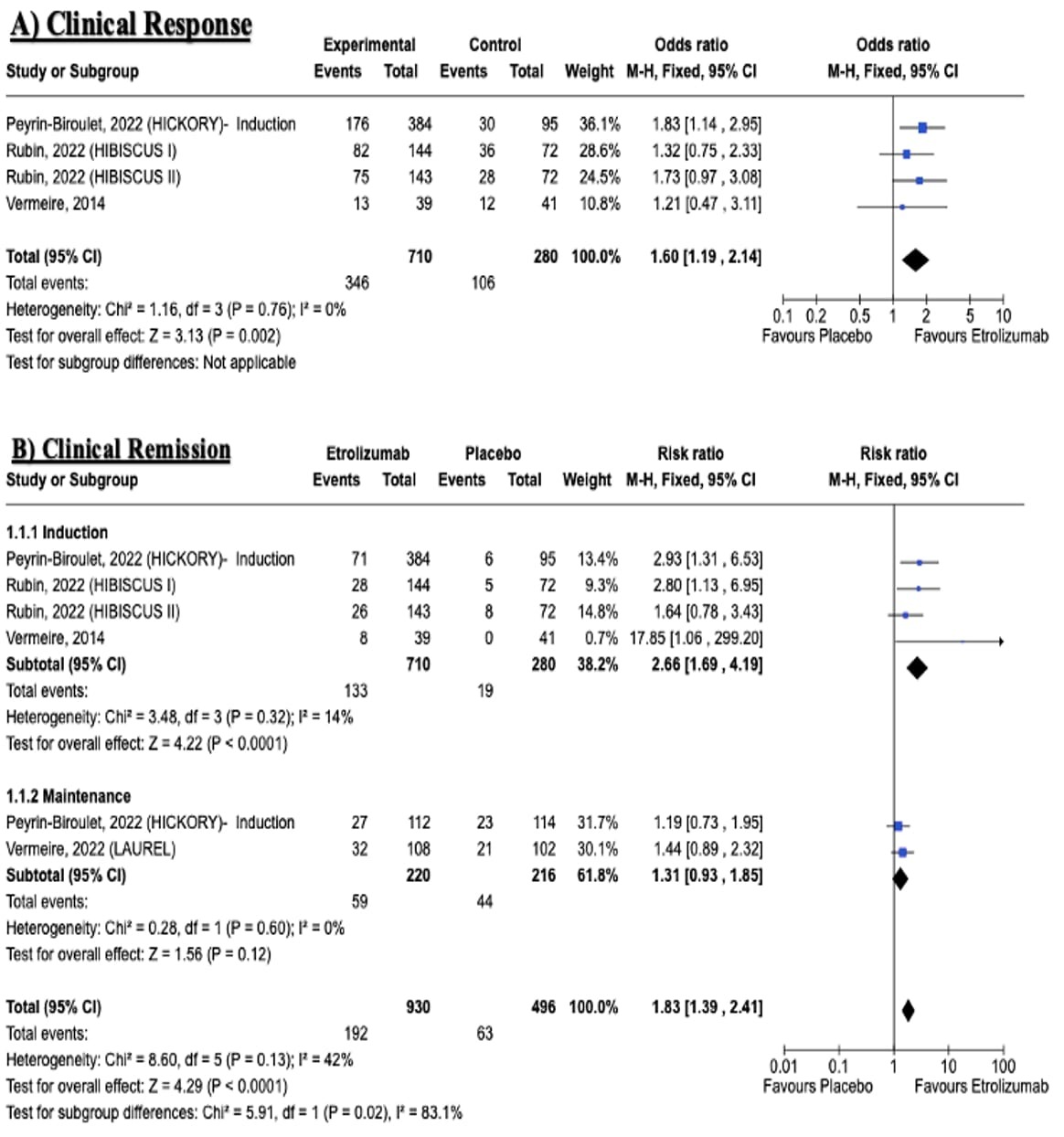
Results: Five RCTs with a total of 1120 patients were included (Table 1). Etrolizumab was associated with a significantly increased clinical response (RR: 1.60, 95% CI [1.19, 2.14], P = 0.002) and clinical remission rates during both induction (RR: 1.83, 95% CI [1.39, 2.41], P < 0.0001) and maintenance phases (RR: 2.66, 95% CI [1.69, 4.19], P < 0.0001) (Figure-1). It also improved endoscopic outcomes, including endoscopic improvement (induction: RR: 1.55, 95% CI [1.29, 1.87], P < 0.0001; maintenance: RR: 1.69, 95% CI [1.24, 2.30], P = 0.0008) and endoscopic remission (induction: RR: 2.07, 95% CI [1.54, 2.80], P < 0.0001; maintenance: RR: 1.92, 95% CI [1.29, 2.85], P = 0.001), along with histological remission rates (induction: RR: 1.73, 95% CI [1.37, 2.17], P < 0.00001; maintenance: RR: 2.04, 95% CI [1.40, 2.98], P = 0.0002). No significant differences were observed in glucocorticoids-free remission (RR: 1.94, 95% CI [0.95, 3.94], P = 0.07) or remission maintenance (RR: 1.07, 95% CI [0.60, 1.91], P = 0.81) between etrolizumab and control groups. Safety profiles were comparable between etrolizumab and placebo groups with no significant differences in adverse events or serious adverse events leading to study treatment discontinuation or death.
Discussion: Our analysis suggests that etrolizumab is both safe and effective as an induction and maintenance therapy in the management of moderate to severe UC.
Note: The table for this abstract can be viewed in the ePoster Gallery section of the ACG 2024 ePoster Site or in The American Journal of Gastroenterology's abstract supplement issue, both of which will be available starting October 27, 2024.
Disclosures:
Fouad Jaber, MD, MS1, Saqr Alsakarneh, MD1, Mohammed Ayyad, MD2, Tala Alsharaeh, MD3, Ahmed-Jordan Salahat, MD3, Mohammad Jaber, MD4, Yassine Kilani, MD5, Mohammad Aldiabat, MD6, Manesh Kumar Gangwani, MD7, Yazan Abboud, MD2, Ahmed Fares, MD8, Dushyant S. Dahiya, MD9, Mohamed Ahmed, MD10, Yazan Sallam, MD11, Richa Shukla, MD12. P4339 - Efficacy and Safety of Etrolizumab in the Treatment of Moderate to Severe Ulcerative Colitis: A Systematic Review and Meta-Analysis of Randomized Controlled Trials, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
1University of Missouri - Kansas City School of Medicine, Kansas City, MO; 2Rutgers New Jersey Medical School, Newark, NJ; 3The University of Jordan, Amman, 'Amman, Jordan; 4Al-Azhar University, Gaza, Palestinian Territories; 5SSM Health Saint Louis University Hospital, St. Louis, MO; 6Washington University in St. Louis, St. Louis, MO; 7University of Toledo, Toledo, OH; 8Tufts Medical Center, Boston, MA; 9The University of Kansas School of Medicine, Kansas City, KS; 10University of Missouri-Kansas City, Saint Luke's Hospital, Kansas City, MO; 11University of Missouri, Kansas City, MO; 12Baylor College of Medicine, Houston, TX
Introduction: Etrolizumab is not yet commercially available for UC treatment. Despite the promising outcomes and safety profile of etrolizumab owing to its selective mechanism of action, the certainty behind its efficacy in the treatment of moderate to severe UC remains unclear. Our study aims to evaluate the effectiveness and safety of etrolizumab, a monoclonal antibody targeting integrin molecules, particularly the β7 subunit in α4β7 and αEβ7 integrin heterodimers, for the treatment of moderate to severe ulcerative colitis (UC).
Methods: We conducted a systematic review and meta-analysis, gathering randomized controlled trials (RCTs) from PubMed, Web of Science, SCOPUS, and Cochrane up to January 7th, 2023. Using a fixed-effect model, we pooled dichotomous data with RR and 95% CI for analysis.
Results: Five RCTs with a total of 1120 patients were included (Table 1). Etrolizumab was associated with a significantly increased clinical response (RR: 1.60, 95% CI [1.19, 2.14], P = 0.002) and clinical remission rates during both induction (RR: 1.83, 95% CI [1.39, 2.41], P < 0.0001) and maintenance phases (RR: 2.66, 95% CI [1.69, 4.19], P < 0.0001) (Figure-1). It also improved endoscopic outcomes, including endoscopic improvement (induction: RR: 1.55, 95% CI [1.29, 1.87], P < 0.0001; maintenance: RR: 1.69, 95% CI [1.24, 2.30], P = 0.0008) and endoscopic remission (induction: RR: 2.07, 95% CI [1.54, 2.80], P < 0.0001; maintenance: RR: 1.92, 95% CI [1.29, 2.85], P = 0.001), along with histological remission rates (induction: RR: 1.73, 95% CI [1.37, 2.17], P < 0.00001; maintenance: RR: 2.04, 95% CI [1.40, 2.98], P = 0.0002). No significant differences were observed in glucocorticoids-free remission (RR: 1.94, 95% CI [0.95, 3.94], P = 0.07) or remission maintenance (RR: 1.07, 95% CI [0.60, 1.91], P = 0.81) between etrolizumab and control groups. Safety profiles were comparable between etrolizumab and placebo groups with no significant differences in adverse events or serious adverse events leading to study treatment discontinuation or death.
Discussion: Our analysis suggests that etrolizumab is both safe and effective as an induction and maintenance therapy in the management of moderate to severe UC.

Figure: Figure 1: Forest plots for efficacy outcomes (A: Clinical response, B: Clinical remission)
Note: The table for this abstract can be viewed in the ePoster Gallery section of the ACG 2024 ePoster Site or in The American Journal of Gastroenterology's abstract supplement issue, both of which will be available starting October 27, 2024.
Disclosures:
Fouad Jaber indicated no relevant financial relationships.
Saqr Alsakarneh indicated no relevant financial relationships.
Mohammed Ayyad indicated no relevant financial relationships.
Tala Alsharaeh indicated no relevant financial relationships.
Ahmed-Jordan Salahat indicated no relevant financial relationships.
Mohammad Jaber indicated no relevant financial relationships.
Yassine Kilani indicated no relevant financial relationships.
Mohammad Aldiabat indicated no relevant financial relationships.
Manesh Kumar Gangwani indicated no relevant financial relationships.
Yazan Abboud indicated no relevant financial relationships.
Ahmed Fares indicated no relevant financial relationships.
Dushyant Dahiya indicated no relevant financial relationships.
Mohamed Ahmed indicated no relevant financial relationships.
Yazan Sallam indicated no relevant financial relationships.
Richa Shukla: Abbvie – Speakers Bureau.
Fouad Jaber, MD, MS1, Saqr Alsakarneh, MD1, Mohammed Ayyad, MD2, Tala Alsharaeh, MD3, Ahmed-Jordan Salahat, MD3, Mohammad Jaber, MD4, Yassine Kilani, MD5, Mohammad Aldiabat, MD6, Manesh Kumar Gangwani, MD7, Yazan Abboud, MD2, Ahmed Fares, MD8, Dushyant S. Dahiya, MD9, Mohamed Ahmed, MD10, Yazan Sallam, MD11, Richa Shukla, MD12. P4339 - Efficacy and Safety of Etrolizumab in the Treatment of Moderate to Severe Ulcerative Colitis: A Systematic Review and Meta-Analysis of Randomized Controlled Trials, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
