Tuesday Poster Session
Category: IBD
P4341 - Mirikizumab Improves Quality of Life in Patients With Moderately to Severely Active Crohn’s Disease: Results from the Phase 3 VIVID-1 Study
Tuesday, October 29, 2024
10:30 AM - 4:00 PM ET
Location: Exhibit Hall E

Has Audio

Scott D. Lee, MD
Digestive Health Center, University of Washington Medical Center
Seattle, WA
Presenting Author(s)
Scott D. Lee, MD1, Séverine Vermeire, MD, PhD2, Ryan C. Ungaro, MD3, Frederick Durand, 4, Nathan Morris, 4, Guanglei Yu, 4, Aisha Vadhariya, 4, Kristina Traxler, 4, Deborah A. Fisher, MD, MHS5, Millie D. Long, MD, MPH6
1Digestive Health Center, University of Washington Medical Center, Seattle, WA; 2University Hospitals Leuven, Leuven, Vlaams-Brabant, Belgium; 3Icahn School of Medicine at Mount Sinai, New York, NY; 4Eli Lilly and Company, Indianapolis, IN; 5Eli Lilly and Company, Raleigh, NC; 6Center for Gastrointestinal Biology and Disease, University of North Carolina, Chapel Hill, NC
Introduction: Mirikizumab, an anti-IL-23p19 antibody, has demonstrated significant efficacy versus placebo (PBO) in patients with Crohn’s disease (CD) in the Phase 3, randomized, double-blind, treat-through VIVID-1 study (NCT03926130).1,2 Here we present the effect of mirikizumab versus PBO on health-related quality of life in the VIVID-1 study.
Methods: Adult patients (N=1065) with moderately to severely active CD were randomized (6:3:2) to receive mirikizumab (N=579), ustekinumab (N=287), or PBO (N=199). At Week (W)12, PBO responders continued to PBO, while PBO non-responders received the blinded mirikizumab regimen described above. Effect of mirikizumab on health-related quality of life was assessed at W12 and W52 with change from baseline in: (1) Short Form-36 Health Survey Version 2 (SF-36), physical (PCS) and mental component summaries (MCS), and domain scores and (2) EQ-5D-5L visual analogue scale (VAS). SF-36 PCS and MCS minimal clinically important difference (MCID) response (≥5-point improvement from baseline) rates were also evaluated at W12 and W52.3 Treatments were compared using analysis of covariance (continuous endpoints) and Cochran–Mantel–Haenszel test (binary endpoints). PBO non-responders switched to mirikizumab remained in the PBO group and their data after W12 were imputed using modified baseline observation carried forward for continuous endpoints and non-responder imputation for binary endpoints.
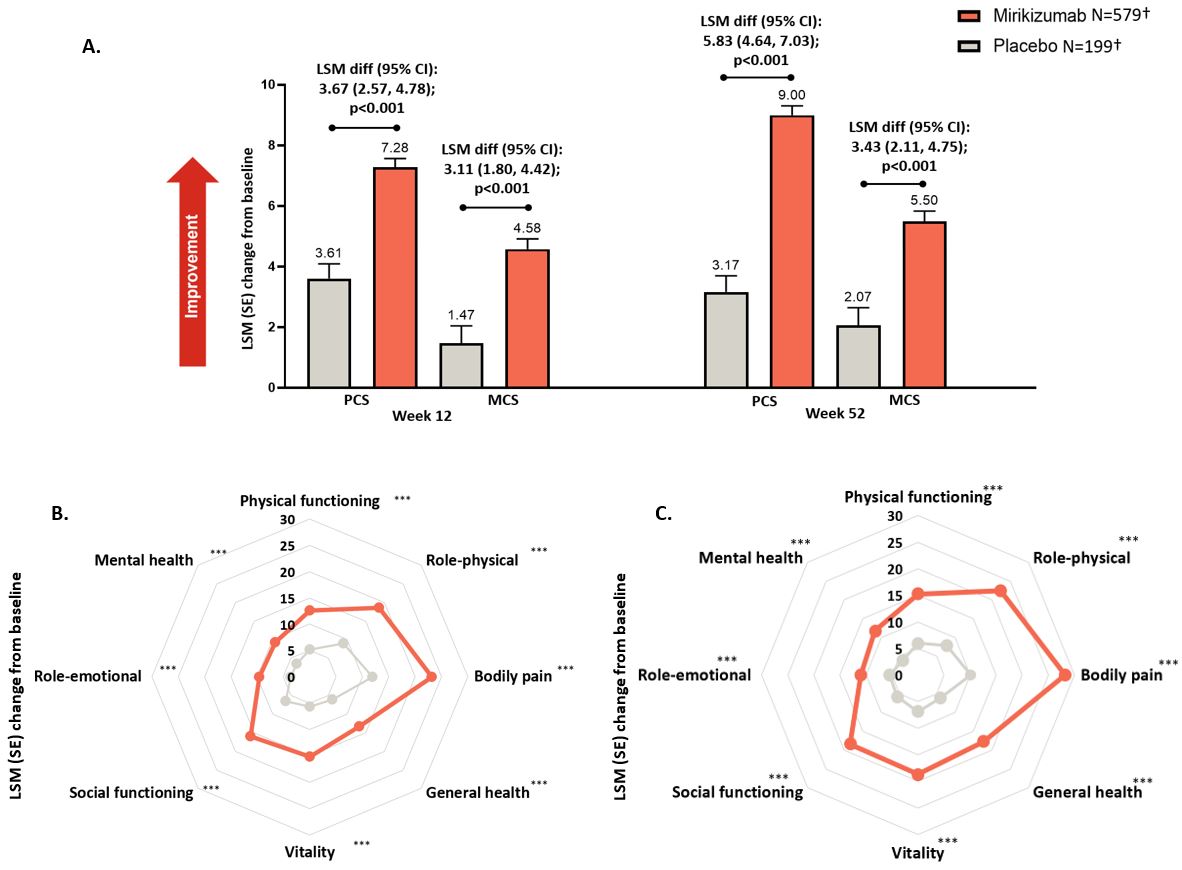
Results: Significant improvements in mean change from baseline to W12 and W52 were observed with mirikizumab versus PBO for SF-36 summary and domain scores (all p< 0.001; Figure). Similarly, a greater improvement was observed with mirikizumab versus PBO for mean change in EQ-5D-5L VAS scores from baseline to W12 and W52 (both p< 0.001). At W12 and W52, a greater proportion of mirikizumab-treated patients versus PBO achieved both SF-36 PCS and MCS MCID response (p< 0.001).
Discussion: In the VIVID-1 study, patients with moderately to severely active CD treated with mirikizumab achieved nominally statistically significant and clinically important improvements in health-related quality of life measures compared to PBO at W12 and W52.
1. Ferrante M, et al. J Crohns Colitis. 2024;18(Supplement_1):i7-9.
2. Jairath V, et al. J Crohns Colitis. 2024;18(Supplement_1):i62-4.
3. Coteur G, et al. Aliment Pharmacol Ther. 2009;29(9):1032-41.
Disclosures:
Scott D. Lee, MD1, Séverine Vermeire, MD, PhD2, Ryan C. Ungaro, MD3, Frederick Durand, 4, Nathan Morris, 4, Guanglei Yu, 4, Aisha Vadhariya, 4, Kristina Traxler, 4, Deborah A. Fisher, MD, MHS5, Millie D. Long, MD, MPH6. P4341 - Mirikizumab Improves Quality of Life in Patients With Moderately to Severely Active Crohn’s Disease: Results from the Phase 3 VIVID-1 Study, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
1Digestive Health Center, University of Washington Medical Center, Seattle, WA; 2University Hospitals Leuven, Leuven, Vlaams-Brabant, Belgium; 3Icahn School of Medicine at Mount Sinai, New York, NY; 4Eli Lilly and Company, Indianapolis, IN; 5Eli Lilly and Company, Raleigh, NC; 6Center for Gastrointestinal Biology and Disease, University of North Carolina, Chapel Hill, NC
Introduction: Mirikizumab, an anti-IL-23p19 antibody, has demonstrated significant efficacy versus placebo (PBO) in patients with Crohn’s disease (CD) in the Phase 3, randomized, double-blind, treat-through VIVID-1 study (NCT03926130).1,2 Here we present the effect of mirikizumab versus PBO on health-related quality of life in the VIVID-1 study.
Methods: Adult patients (N=1065) with moderately to severely active CD were randomized (6:3:2) to receive mirikizumab (N=579), ustekinumab (N=287), or PBO (N=199). At Week (W)12, PBO responders continued to PBO, while PBO non-responders received the blinded mirikizumab regimen described above. Effect of mirikizumab on health-related quality of life was assessed at W12 and W52 with change from baseline in: (1) Short Form-36 Health Survey Version 2 (SF-36), physical (PCS) and mental component summaries (MCS), and domain scores and (2) EQ-5D-5L visual analogue scale (VAS). SF-36 PCS and MCS minimal clinically important difference (MCID) response (≥5-point improvement from baseline) rates were also evaluated at W12 and W52.3 Treatments were compared using analysis of covariance (continuous endpoints) and Cochran–Mantel–Haenszel test (binary endpoints). PBO non-responders switched to mirikizumab remained in the PBO group and their data after W12 were imputed using modified baseline observation carried forward for continuous endpoints and non-responder imputation for binary endpoints.
Results: Significant improvements in mean change from baseline to W12 and W52 were observed with mirikizumab versus PBO for SF-36 summary and domain scores (all p< 0.001; Figure). Similarly, a greater improvement was observed with mirikizumab versus PBO for mean change in EQ-5D-5L VAS scores from baseline to W12 and W52 (both p< 0.001). At W12 and W52, a greater proportion of mirikizumab-treated patients versus PBO achieved both SF-36 PCS and MCS MCID response (p< 0.001).
Discussion: In the VIVID-1 study, patients with moderately to severely active CD treated with mirikizumab achieved nominally statistically significant and clinically important improvements in health-related quality of life measures compared to PBO at W12 and W52.
1. Ferrante M, et al. J Crohns Colitis. 2024;18(Supplement_1):i7-9.
2. Jairath V, et al. J Crohns Colitis. 2024;18(Supplement_1):i62-4.
3. Coteur G, et al. Aliment Pharmacol Ther. 2009;29(9):1032-41.

Figure: Figure. Change from baseline in SF-36 summary and domain scores at Week 12 and Week 52 in the Primary Analysis Set
A. SF-36 PCS and MCS Summary scores
B. SF-36 domain scores at Week 12
C. SF-36 domain scores at Week 52
†Adult patients (N=1065) with moderately to severely active CD were randomized (6:3:2) to receive mirikizumab (N=579), ustekinumab (N=287), or PBO (N=199); n=576 mirikizumab and n=198 PBO patients had baseline data and were included in the analysis. After Week 12, placebo-responders continued placebo; placebo-nonresponders switched to mirikizumab, remained included in the placebo group and baseline values were carried forward to derive the change from baseline at Week 52. Results for mirikizumab versus the ustekinumab arm from VIVID-1 study are presented elsewhere.2 Least squares mean (SE) changes from baseline were compared using analysis of covariance with modified baseline observation carried forward.
Increase in SF-36 (range 0-100) scores indicate improvement.
Reported p-values are unadjusted for multiple testing.
*** represents p<0.001. p-value represents pairwise treatment comparison between mirikizumab versus placebo.
Abbreviations: CI, confidence interval; diff, difference; LSM = Least squares mean; MCS = Mental Component Summary; N=number of patients in the analysis population; n=number of patients with baseline value; PCS = Physical Component Summary; SE = standard error; SF-36 = Short Form-36 Health Survey Version 2 form
A. SF-36 PCS and MCS Summary scores
B. SF-36 domain scores at Week 12
C. SF-36 domain scores at Week 52
†Adult patients (N=1065) with moderately to severely active CD were randomized (6:3:2) to receive mirikizumab (N=579), ustekinumab (N=287), or PBO (N=199); n=576 mirikizumab and n=198 PBO patients had baseline data and were included in the analysis. After Week 12, placebo-responders continued placebo; placebo-nonresponders switched to mirikizumab, remained included in the placebo group and baseline values were carried forward to derive the change from baseline at Week 52. Results for mirikizumab versus the ustekinumab arm from VIVID-1 study are presented elsewhere.2 Least squares mean (SE) changes from baseline were compared using analysis of covariance with modified baseline observation carried forward.
Increase in SF-36 (range 0-100) scores indicate improvement.
Reported p-values are unadjusted for multiple testing.
*** represents p<0.001. p-value represents pairwise treatment comparison between mirikizumab versus placebo.
Abbreviations: CI, confidence interval; diff, difference; LSM = Least squares mean; MCS = Mental Component Summary; N=number of patients in the analysis population; n=number of patients with baseline value; PCS = Physical Component Summary; SE = standard error; SF-36 = Short Form-36 Health Survey Version 2 form
Disclosures:
Scott Lee: AbbVie – Consultant. Bristol Myers Squibb – Consultant. Eli Lilly – Consultant. Janssen – Consultant.
Séverine Vermeire: AbbVie – Consultant, Grant/Research Support. Abivax – Consultant. AbolerlsPharma – Consultant. AgomAb – Consultant. Alimentiv – Consultant. Arena Pharmaceuticals – Consultant. AstraZeneca – Consultant. BioraTherapeutics – Consultant. Boehringer Ingelheim – Consultant. Bristol Myers Squibb – Consultant. Celgene – Consultant. Cytoki Pharma – Consultant. Dr Falk Pharma – Consultant. Eli Lilly – Consultant. Ferring – Consultant. Galapagos – Consultant, Grant/Research Support. Genentech Roche – Consultant. Gilead – Consultant. GSK – Consultant. Hospira – Consultant. J&J – Consultant, Grant/Research Support. Janssen – Consultant. lmidomics – Consultant. Materia Prima – Consultant. Mestag Therapeutics – Consultant. Microbiotica – Consultant. MiroBio – Consultant. Morphic – Consultant. MrMHealth – Consultant. MSD – Consultant. Mundipharma – Consultant. Pfizer Inc – Consultant, Grant/Research Support. Prodigest – Consultant. Progenity – Consultant. Prometheus – Consultant. Robarts Clinical Trials – Consultant. Surrozen – Consultant. Takeda – Consultant, Grant/Research Support. Theravance – Consultant. Tillots Pharma AG – Consultant. VectivBio – Consultant. Ventyx – Consultant. Zealand Pharma – Consultant.
Ryan C. Ungaro: AbbVie – Advisory Committee/Board Member, Grant/Research Support. Boehringer Ingelheim – Grant/Research Support. Bristol Myers Squibb – Advisory Committee/Board Member. Celltrion – Advisory Committee/Board Member, Consultant. Eli Lilly – Grant/Research Support. Inotrem – Advisory Committee/Board Member, Consultant. Janssen – Advisory Committee/Board Member. Pfizer – Advisory Committee/Board Member, Grant/Research Support. Prometheus Labratories – Grant/Research Support. Roivant – Advisory Committee/Board Member, Consultant. Takeda – Advisory Committee/Board Member.
Frederick Durand: Eli Lilly and Company – Employee, Stock Options.
Nathan Morris: Eli Lilly and Company – Employee, Stock Options.
Guanglei Yu: Eli Lilly and Company – Employee, Stock Options.
Aisha Vadhariya: Eli Lilly and Company – Employee, Stock Options.
Kristina Traxler: Eli Lilly and Company – Employee, Stock Options.
Deborah Fisher: Eli Lilly and Company – Employee, Stock Options.
Millie Long: AbbVie – Consultant. Bristol-Myers Squibb – Consultant. Eli Lilly – Consultant, Grant/Research Support. Intercept – Consultant. Janssen – Consultant. Pfizer Inc – Consultant, Grant/Research Support. Prometheus – Consultant. Roche – Consultant. Roivant – Consultant. Takeda – Consultant, Grant/Research Support. Target RWE – Consultant.
Scott D. Lee, MD1, Séverine Vermeire, MD, PhD2, Ryan C. Ungaro, MD3, Frederick Durand, 4, Nathan Morris, 4, Guanglei Yu, 4, Aisha Vadhariya, 4, Kristina Traxler, 4, Deborah A. Fisher, MD, MHS5, Millie D. Long, MD, MPH6. P4341 - Mirikizumab Improves Quality of Life in Patients With Moderately to Severely Active Crohn’s Disease: Results from the Phase 3 VIVID-1 Study, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
