Tuesday Poster Session
Category: IBD
P4352 - Histologic and Combined Histologic and Endoscopic Outcomes After Guselkumab Maintenance Therapy in Patients With Moderately to Severely Active Ulcerative Colitis: Week 44 Results From the Phase 3 QUASAR Maintenance Study
Tuesday, October 29, 2024
10:30 AM - 4:00 PM ET
Location: Exhibit Hall E
Has Audio

Bruce E. Sands, MD, FACG
Icahn School of Medicine at Mount Sinai
New York, NY
Presenting Author(s)
Julián Panés, MD1, Axel Dignass, MD, PhD2, Tadakazu Hisamatsu, MD, PhD3, Shadi Yarandi, PhD4, Kuan-Hsiang G. Huang, MD, PhD5, Matthew Germinaro, MD5, Sunandini Sridhar, PhD4, Patrick Branigan, BS4, Rebbecca Wilson, 6, Hongyan Zhang, PhD5, Fernando Magro, MD, PhD7, Vipul Jairath, MBChB8, Brian G.. Feagan, MD8, Gary R. Lichtenstein, MD, FACG9, David T. Rubin, MD, FACG10, Bruce E.. Sands, MD, FACG11
1Hospital Clínic de Barcelona, IDIBAPS, CIBERehd, Barcelona, Catalonia, Spain; 2Agaplesion Markus Hospital, Goethe University, Frankfurt, Hessen, Germany; 3Kyorin University School of Medicine, Tokyo, Tokyo, Japan; 4Janssen Research & Development, LLC, Spring House, PA; 5Janssen Research and Development, Spring House, PA; 6Janssen Research and Development, LLC, Spring House, PA; 7University of Porto and Centro Hospitalar, Porto, Porto, Portugal; 8Western University, London, ON, Canada; 9Perelman Center for Advanced Medicine, University of Pennsylvania, Philadelphia, PA; 10University of Chicago Medicine Inflammatory Bowel Disease Center, Chicago, IL; 11Icahn School of Medicine at Mount Sinai, New York, NY
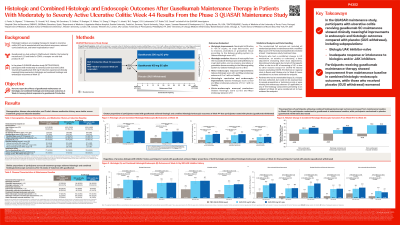
Introduction: The phase 3 QUASAR maintenance study (NCT04033445) evaluated the efficacy and safety of guselkumab (GUS), a dual-acting IL-23p19 subunit inhibitor that potently neutralizes IL-23, in patients (pts) who achieved clinical response to 12 weeks of IV GUS. Here we present results for effects of GUS maintenance on histologic and combined histologic and endoscopic outcomes at Week (Wk) 44.
Methods: At maintenance baseline (BL), clinical responders following 12 weeks of GUS IV induction from the QUASAR Phase 2b and 3 induction studies were randomized 1:1:1 to GUS 200 mg SC q4w, GUS 100 mg SC q8w, or GUS withdrawal (PBO SC). Colonic biopsies were collected during endoscopy at maintenance BL and Wk 44 to evaluate treatment effect on histology measured using Geboes, Robarts, and Nancy Histological Index. Histologic improvement, histologic remission, the combination of histologic improvement and endoscopic improvement (histo-endoscopic mucosal improvement; HEMI), the combination of histologic remission and endoscopic improvement, and the combination of histologic remission and endoscopic remission (histo-endoscopic mucosal remission) were evaluated at Wk 44 (see Table for definitions).
Results: Of the 568 pts randomized (at induction BL: mean age, 40.7yrs; mean UC disease duration, 7.8yrs; mean modified Mayo score, 6.9 [63.9% with severe disease]; Mayo endoscopy subscore of 3, 66.4%), 190 were receiving GUS 200 mg q4w, 188 GUS 100 mg q8w, and 190 PBO. BL characteristics were similar across treatment groups. Histologic activity at maintenance BL was similar for the GUS 200 mg q4w, GUS 100 mg q8w, and PBO treatment groups (mean continuous Geboes total score: 6.7, 6.8, and 6.9, respectively).
Improvements in histologic activity at Wk 44 were observed in pts treated with GUS 200 mg q4w and GUS 100 mg q8w, while pts assigned to PBO worsened. At Wk 44, greater proportions of pts treated with GUS 200 mg q4w and GUS 100 mg q8w achieved the assessed endpoints compared to PBO (Table). Across biologic/JAK inhibitor therapy history subpopulations, greater proportions of GUS-treated pts achieved the assessed endpoints compared to PBO.
Discussion: In this phase 3 maintenance study, pts with UC treated with GUS 200 mg SC q4w or GUS 100 mg SC q8w experienced clinically meaningful improvements in histologic and combined histologic and endoscopic outcomes at Wk 44 compared to PBO-treated pts.
Note: The table for this abstract can be viewed in the ePoster Gallery section of the ACG 2024 ePoster Site or in The American Journal of Gastroenterology's abstract supplement issue, both of which will be available starting October 27, 2024.
Disclosures:
Julián Panés, MD1, Axel Dignass, MD, PhD2, Tadakazu Hisamatsu, MD, PhD3, Shadi Yarandi, PhD4, Kuan-Hsiang G. Huang, MD, PhD5, Matthew Germinaro, MD5, Sunandini Sridhar, PhD4, Patrick Branigan, BS4, Rebbecca Wilson, 6, Hongyan Zhang, PhD5, Fernando Magro, MD, PhD7, Vipul Jairath, MBChB8, Brian G.. Feagan, MD8, Gary R. Lichtenstein, MD, FACG9, David T. Rubin, MD, FACG10, Bruce E.. Sands, MD, FACG11. P4352 - Histologic and Combined Histologic and Endoscopic Outcomes After Guselkumab Maintenance Therapy in Patients With Moderately to Severely Active Ulcerative Colitis: Week 44 Results From the Phase 3 QUASAR Maintenance Study, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
1Hospital Clínic de Barcelona, IDIBAPS, CIBERehd, Barcelona, Catalonia, Spain; 2Agaplesion Markus Hospital, Goethe University, Frankfurt, Hessen, Germany; 3Kyorin University School of Medicine, Tokyo, Tokyo, Japan; 4Janssen Research & Development, LLC, Spring House, PA; 5Janssen Research and Development, Spring House, PA; 6Janssen Research and Development, LLC, Spring House, PA; 7University of Porto and Centro Hospitalar, Porto, Porto, Portugal; 8Western University, London, ON, Canada; 9Perelman Center for Advanced Medicine, University of Pennsylvania, Philadelphia, PA; 10University of Chicago Medicine Inflammatory Bowel Disease Center, Chicago, IL; 11Icahn School of Medicine at Mount Sinai, New York, NY
Introduction: The phase 3 QUASAR maintenance study (NCT04033445) evaluated the efficacy and safety of guselkumab (GUS), a dual-acting IL-23p19 subunit inhibitor that potently neutralizes IL-23, in patients (pts) who achieved clinical response to 12 weeks of IV GUS. Here we present results for effects of GUS maintenance on histologic and combined histologic and endoscopic outcomes at Week (Wk) 44.
Methods: At maintenance baseline (BL), clinical responders following 12 weeks of GUS IV induction from the QUASAR Phase 2b and 3 induction studies were randomized 1:1:1 to GUS 200 mg SC q4w, GUS 100 mg SC q8w, or GUS withdrawal (PBO SC). Colonic biopsies were collected during endoscopy at maintenance BL and Wk 44 to evaluate treatment effect on histology measured using Geboes, Robarts, and Nancy Histological Index. Histologic improvement, histologic remission, the combination of histologic improvement and endoscopic improvement (histo-endoscopic mucosal improvement; HEMI), the combination of histologic remission and endoscopic improvement, and the combination of histologic remission and endoscopic remission (histo-endoscopic mucosal remission) were evaluated at Wk 44 (see Table for definitions).
Results: Of the 568 pts randomized (at induction BL: mean age, 40.7yrs; mean UC disease duration, 7.8yrs; mean modified Mayo score, 6.9 [63.9% with severe disease]; Mayo endoscopy subscore of 3, 66.4%), 190 were receiving GUS 200 mg q4w, 188 GUS 100 mg q8w, and 190 PBO. BL characteristics were similar across treatment groups. Histologic activity at maintenance BL was similar for the GUS 200 mg q4w, GUS 100 mg q8w, and PBO treatment groups (mean continuous Geboes total score: 6.7, 6.8, and 6.9, respectively).
Improvements in histologic activity at Wk 44 were observed in pts treated with GUS 200 mg q4w and GUS 100 mg q8w, while pts assigned to PBO worsened. At Wk 44, greater proportions of pts treated with GUS 200 mg q4w and GUS 100 mg q8w achieved the assessed endpoints compared to PBO (Table). Across biologic/JAK inhibitor therapy history subpopulations, greater proportions of GUS-treated pts achieved the assessed endpoints compared to PBO.
Discussion: In this phase 3 maintenance study, pts with UC treated with GUS 200 mg SC q4w or GUS 100 mg SC q8w experienced clinically meaningful improvements in histologic and combined histologic and endoscopic outcomes at Wk 44 compared to PBO-treated pts.
Note: The table for this abstract can be viewed in the ePoster Gallery section of the ACG 2024 ePoster Site or in The American Journal of Gastroenterology's abstract supplement issue, both of which will be available starting October 27, 2024.
Disclosures:
Julián Panés: AbbVie – Consultant, Honorarium. Alimentiv – Advisory Committee/Board Member, Consultant, Honorarium. Athos – Consultant, Honorarium. Atomwise – Consultant, Honorarium. Boehringer Ingelheim – Consultant, Honorarium. Celsius – Consultant, Honorarium. Ferring – Consultant, Honorarium. Galapagos – Consultant, Honorarium. Genentech/Roche – Consultant, Honorarium. GlaxoSmithKline – Consultant, Honorarium. Janssen – Consultant, Honorarium. Mirum – Advisory Committee/Board Member, Consultant, honorarium. Nimbus – Consultant, Honorarium. Pfizer – Consultant, Honorarium. Progenity – Consultant, Honorarium. Prometheus – Consultant, Honorarium. Protagonist – Consultant, Honorarium. Revolo – Consultant, Honorarium. Sanofi – Advisory Committee/Board Member, Consultant, Honorarium. Sorriso – Advisory Committee/Board Member, Consultant, Honorarium. Surrozen – Advisory Committee/Board Member, Consultant, Honorarium. Takeda – Consultant, Honorarium. Wasserman – Consultant, Honorarium.
Axel Dignass: AbbVie – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Payment for manuscript preparation, Speakers Bureau. Abivax – Advisor or Review Panel Member, Advisory Committee/Board Member. Amgen – Consultant. Arena Pharmaceuticals – Consultant. Biogen – Consultant, Speakers Bureau. Boehringer Ingelheim – Consultant. Bristol Myers Squibb – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant. CED Service GmbH – Speakers Bureau. Celltrion – Consultant, Speakers Bureau. Dr Falk Foundation – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Payment for manuscript preparation, Speakers Bureau. Ferring Pharmaceuticals – Consultant, Speakers Bureau. Fresenius Kabi – Consultant. Galapagos – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Speakers Bureau. Gilead – Advisor or Review Panel Member, Advisory Committee/Board Member, Speakers Bureau. High5MD – Speakers Bureau. Janssen – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Payment for manuscript preparation, Speakers Bureau. Lilly – Consultant. Materia Prima – Speakers Bureau. MedToday – Speakers Bureau. MSD – Consultant, Speakers Bureau. Pfizer – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Speakers Bureau. Pharmacosmos – Consultant. Roche – Consultant. Sandoz – Consultant, Speakers Bureau. Stada – Consultant. Takeda – Consultant, Payment for manuscript preparation, Speakers Bureau. Thieme – Payment for manuscript preparation. Tillotts – Consultant, Speakers Bureau. UniMed Verlag – Payment for manuscript preparation. Vifor Pharma – Consultant, Speakers Bureau.
Tadakazu Hisamatsu: AbbVie – Grant/Research Support, lecture fees. Bristol Myers Squibb – Consultant. Daiichi-Sankyo – Grant/Research Support. EA Pharma – Consultant, Grant/Research Support, lecture fees. Gilead Sciences – Consultant. Janssen – Consultant. JIMRO – Grant/Research Support. Mitsubishi Tanabe Pharma Corporation – Grant/Research Support, lecture fees. Mochida Pharmaceutical – Grant/Research Support. Nippon Kayaku – Grant/Research Support. Pfizer – Grant/Research Support. Takeda Pharmaceutical – Grant/Research Support, lecture fees.
Shadi Yarandi: Johnson & Johnson – Employee, Stock Options, Stock-publicly held company(excluding mutual/index funds).
Kuan-Hsiang G. Huang: Johnson & Johnson – Employee, Stock Options, Stock-publicly held company(excluding mutual/index funds).
Matthew Germinaro: Johnson & Johnson – Employee, Stock Options, Stock-publicly held company(excluding mutual/index funds).
Sunandini Sridhar: Johnson & Johnson – Employee, Stock Options, Stock-publicly held company(excluding mutual/index funds).
Patrick Branigan: Johnson & Johnson – Employee, Stock Options, Stock-publicly held company(excluding mutual/index funds).
Rebbecca Wilson: Johnson & Johnson – Employee, Stock Options, Stock-publicly held company(excluding mutual/index funds).
Hongyan Zhang: Johnson & Johnson – Employee, Stock Options, Stock-publicly held company(excluding mutual/index funds).
Fernando Magro indicated no relevant financial relationships.
Vipul Jairath: AbbVie – Consultant, Grant/Research Support, Speakers Bureau. Alimentiv – Consultant, Employee, Grant/Research Support, Speakers Bureau. Arena Pharmaceuticals – Consultant, Grant/Research Support, Speakers Bureau. Asahi Kasei Pharma – Consultant, Grant/Research Support, Speakers Bureau. Asieris Pharmaceuticals – Consultant, Grant/Research Support, Speakers Bureau. AstraZeneca – Consultant, Grant/Research Support, Speakers Bureau. Avoro Capital – Advisory Committee/Board Member, Consultant. Bristol Myers Squibb – Consultant, Grant/Research Support, Speakers Bureau. Celltrion – Consultant, Grant/Research Support, Speakers Bureau. Eli Lilly and Company – Consultant, Grant/Research Support, Speakers Bureau. Endpoint Health – Advisory Committee/Board Member, Consultant. Enthera – Advisory Committee/Board Member, Consultant. Ferring Pharmaceuticals – Consultant, Grant/Research Support, Speakers Bureau. Flagship Pioneering – Consultant, Grant/Research Support, Speakers Bureau. Fresenius Kabi – Consultant, Grant/Research Support, Speakers Bureau. Galapagos NV – Consultant, Grant/Research Support, Speakers Bureau. Genentech – Consultant, Grant/Research Support, Speakers Bureau. Gilde Healthcare – Advisory Committee/Board Member, Consultant. Gilead Sciences – Consultant, Grant/Research Support, Speakers Bureau. GlaxoSmithKline – Consultant, Grant/Research Support, Speakers Bureau. Innomar – Advisory Committee/Board Member, Consultant. JAMP – Advisory Committee/Board Member, Consultant. Janssen – Consultant, Grant/Research Support, Speakers Bureau. London Health Sciences Centre – Employee. Merck – Consultant, Grant/Research Support, Speakers Bureau. Metacrine – Consultant, Grant/Research Support, Speakers Bureau. Mylan – Consultant, Grant/Research Support, Speakers Bureau. Pandion Therapeutics – Consultant, Grant/Research Support, Speakers Bureau. Pendopharm – Consultant, Grant/Research Support, Speakers Bureau. Pfizer – Consultant, Grant/Research Support, Speakers Bureau. Prometheus Therapeutics and Diagnostics – Consultant, Grant/Research Support, Speakers Bureau. Protagonist Therapeutics – Consultant, Grant/Research Support, Speakers Bureau. Reistone Biopharma – Consultant, Grant/Research Support, Speakers Bureau. Roche – Consultant, Grant/Research Support, Speakers Bureau. Roivant – Advisory Committee/Board Member, Consultant. Sandoz – Consultant, Grant/Research Support, Speakers Bureau. SCOPE – Advisory Committee/Board Member, Consultant. Second Genome – Consultant, Grant/Research Support, Speakers Bureau. Shire – Speakers Bureau. Sorriso Pharmaceuticals – Consultant, Grant/Research Support, Speakers Bureau. Synedgen – Advisory Committee/Board Member, Consultant. Takeda – Consultant, Grant/Research Support, Speakers Bureau. TD Securities – Advisory Committee/Board Member, Consultant. Teva – Consultant, Grant/Research Support, Speakers Bureau. Topivert – Consultant, Grant/Research Support, Speakers Bureau. Ventyx Biosciences – Consultant, Grant/Research Support, Speakers Bureau. Vividion Therapeutics – Consultant, Grant/Research Support, Speakers Bureau.
Brian Feagan: AbbVie – Advisory Committee/Board Member, Consultant, Speakers Bureau. AbolerIS – Consultant. AgomAB Therapeutics – Consultant. Allianthera – Consultant. Amgen – Advisory Committee/Board Member, Consultant. AnaptysBio – Advisory Committee/Board Member, Consultant. Applied Molecular Transport Inc – Advisory Committee/Board Member, Consultant. Arena Pharma – Consultant. Atomwise – Consultant. Avoro Capital Advisors – Consultant. Axio Research – Advisory Committee/Board Member. BioJamp – Consultant. Biora Therapeutics – Consultant. Boehringer Ingelheim – Advisory Committee/Board Member, Consultant. Boxer – Consultant. Celgene/Bristol Myers Squibb – Advisory Committee/Board Member, Consultant. Celsius Therapeutics – Consultant. Connect BioPharma – Consultant, stock or other ownership interest. Cytoki – Consultant. Disc Medicine – Consultant. Duality – Consultant. EcoR1 Capital – Advisory Committee/Board Member, Consultant. Equillium – Consultant. Ermium – Consultant. First Wave – Consultant. First Word Group – Consultant. Galapagos – Consultant. Galen Atlantica – Consultant. Genentech/Roche – Advisory Committee/Board Member, Consultant. Gilead – Consultant. GlaxoSmithKline – Advisory Committee/Board Member, Consultant. Gossamer Pharma – Consultant, Stock Options. Hinge Bio – Consultant. Hot Spot Therapeutics – Consultant. Imhotex – Consultant. Immunic Therapeutics – Consultant. InDex Pharmaceuticals – Advisory Committee/Board Member, Consultant. JAKAcademy – Consultant. Janssen – Advisory Committee/Board Member, Consultant, Speakers Bureau. Japan Tobacco Inc. – Consultant. Kaleido Biosciences – Consultant. L.E.K. Consulting – Consultant. Landos Biopharma – Consultant. Leadiant – Consultant. Lenczner Slaght – Consultant, payment for expert testimony. LifeSci Capital – Consultant. Lilly – Advisory Committee/Board Member, Consultant. Lument AB – Consultant. Millennium – Consultant. MiroBio – Advisory Committee/Board Member, Consultant. Morgan Lewis – Consultant, payment for expert testimony. Morphic Therapeutics – Advisory Committee/Board Member, Consultant. Mylan – Consultant. OM Pharma – Consultant. Origo BioPharma – Advisory Committee/Board Member, Consultant. Orphagen – Consultant. Pandion Therapeutics – Consultant. Pendopharm – Consultant. Pfizer Inc – Advisory Committee/Board Member, Consultant, Grant/Research Support. Play to Know AG – Consultant. Progenity – Advisory Committee/Board Member, Consultant. Prometheus – Advisory Committee/Board Member, Consultant. Protagonist – Consultant. PTM Therapeutics – Consultant. Q32 Bio – Consultant. Rebiotix – Consultant. REDX – Advisory Committee/Board Member, Consultant. Roche – Consultant. Sandoz – Consultant. Sanofi – Advisory Committee/Board Member, Consultant. Seres Therapeutics – Consultant. Silverback Therapeutics – Consultant. Surrozen Inc. – Consultant. Takeda – Advisory Committee/Board Member, Consultant, Speakers Bureau. Teva – Advisory Committee/Board Member, Consultant. Thelium – Consultant. Tigenix – Consultant. Tillotts Pharma – Advisory Committee/Board Member, Consultant. Ventyx Biosciences – Consultant. VHSquared Ltd – Consultant. Viatris – Consultant. Ysios – Consultant. Ysopia – Consultant. Zealand Pharma – Consultant.
Gary R. Lichtenstein: AbbVie – Consultant. American College of Gastroenterology – honoraria. American Gastroenterological Association – CME. American Regent – Consultant, Honorarium. Celgene – Consultant, Grant/Research Support. Cellceutix – Consultant. Chemed – CME. Eli Lilly – Advisory Committee/Board Member, Consultant. Endo – Consultant. Ferring – Consultant. Gastroenterology & Hepatology – Gastro-Hep Communication, Editor- Honorarium. Gilead – Consultant. IMEDEX – CME. Ironwood – CME. Janssen – Consultant, Grant/Research Support. MedEd Consultants – Consultant. Merck – Consultant, Honorarium. Morphic Therapeutics – Consultant. Pfizer – Consultant, Grant/Research Support. Professional Communications Inc. – Royalties. Prometheus Laboratories – Consultant. Romark – Consultant, honoraria. Salix/Valeant – Consultant. Sandoz – Consultant. Shire – Consultant. SLACK Inc. – Royalties. Springer Science and Business Media – Honorarium. Takeda – Consultant, Grant/Research Support. UCB – Consultant, Grant/Research Support. University of Kentucky – CME. UpToDate – honoraria. Vindico – CME. Virgo – Consultant, Stock Options.
David T. Rubin: AbbVie – Consultant. Altrubio – Consultant. Apex – Consultant. Avalo Therapeutics – Consultant. Boehringer Ingelheim – Consultant. Bristol-Myers Squibb – Consultant. Buhlmann Diagnostics Corp – Consultant. Celgene – Consultant. Connect BioPharma – Consultant. Intouch Group – Consultant. Iterative Health – Consultant. Janssen – Consultant. Lilly – Consultant. Pfizer – Consultant. Samsung Neurologica – Consultant. Takeda – Consultant, Grant/Research Support.
Bruce Sands: AbbVie – Consultant. Abivax – Consultant, Speakers Bureau. Adiso Therapeutics – Consultant. Agomab – Consultant. Alimentiv – Consultant. Amgen – Consultant. AnaptysBio – Consultant. Arena Pharmaceuticals – Consultant. Artugen Therapeutics – Consultant. AstraZeneca – Consultant. Biolojic Design – Consultant. Biora Therapeutics – Consultant. Boehringer Ingelheim – Consultant. Boston Pharmaceuticals – Consultant. Bristol Myers Squibb – Consultant, Grant/Research Support, Other support, Speakers Bureau. Calibr – Consultant. Celgene – Consultant. Celltrion – Consultant. ClostraBio – Consultant. Enthera – Consultant. Envied Biosciences – Consultant. Equilium – Consultant. Evommune – Consultant. Ferring – Consultant. Fiat – Consultant. Fresenius Kabi – Consultant. Galapagos – Consultant. Genentech (Roche) – Consultant. Gilead Sciences – Consultant. Glaxo SmithKline – Consultant. Gossamer Bio – Consultant. Imhotex – Consultant. Index Pharmaceuticals – Consultant. Innovation Pharmaceuticals – Consultant. Inotrem – Consultant. Janssen – Consultant, Grant/Research Support, Other support, Speakers Bureau. Kaleido – Consultant. Kallyope – Consultant. Lilly – Consultant, other support, Speakers Bureau. Merck & Co., Inc., Rahway, NJ, USA – Consultant. Microba – Consultant. Mobius Care – Consultant. Morphic Therapeutics – Consultant. MRM Health – Consultant. Nexus Therapeutics – Consultant. Nimbus Discovery – Consultant. Odyssey Therapeutics – Consultant. Pfizer Inc – Consultant, Grant/Research Support, Other support, Speakers Bureau. Progenity – Consultant. Prometheus Biosciences – Consultant. Prometheus Laboratories – Consultant. Protagonist Therapeutics – Consultant. Q32 Bio – Consultant. Rasayana Therapeutics – Consultant. Recludix Therapeutics – Consultant. Reistone Biopharma – Consultant. Sanofi – Consultant. Spyre Therapeutics – Consultant. Sun Pharma – Consultant. Surrozen – Consultant. Takeda – Consultant, Grant/Research Support, Other support, Speakers Bureau. Target RWE – Consultant. Teva – Consultant. Theravance Biopharma – Consultant, Grant/Research Support. TLL Pharmaceutical – Consultant. Tr1X – Consultant. Union Therapeutics – Consultant. Ventyx Biopharma – Consultant, Stock Options, Stock-publicly held company(excluding mutual/index funds).
Julián Panés, MD1, Axel Dignass, MD, PhD2, Tadakazu Hisamatsu, MD, PhD3, Shadi Yarandi, PhD4, Kuan-Hsiang G. Huang, MD, PhD5, Matthew Germinaro, MD5, Sunandini Sridhar, PhD4, Patrick Branigan, BS4, Rebbecca Wilson, 6, Hongyan Zhang, PhD5, Fernando Magro, MD, PhD7, Vipul Jairath, MBChB8, Brian G.. Feagan, MD8, Gary R. Lichtenstein, MD, FACG9, David T. Rubin, MD, FACG10, Bruce E.. Sands, MD, FACG11. P4352 - Histologic and Combined Histologic and Endoscopic Outcomes After Guselkumab Maintenance Therapy in Patients With Moderately to Severely Active Ulcerative Colitis: Week 44 Results From the Phase 3 QUASAR Maintenance Study, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
