Monday Poster Session
Category: IBD
P2610 - Efficacy and Outcomes of Upadacitinib (Rinvoq) Use in Hospitalized Crohn's Disease Patients
Monday, October 28, 2024
10:30 AM - 4:00 PM ET
Location: Exhibit Hall E
Has Audio
- JB
Jawad Bouhamdan, MD
University of Michigan Health
Ann Arbor, MI
Presenting Author(s)
Jawad Bouhamdan, MD1, Jeffrey A. Berinstein, MD2
1University of Michigan Health, Ann Arbor, MI; 2University of Michigan, Ann Arbor, MI
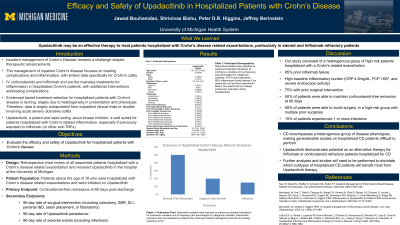
Introduction: Inflammatory bowel disease encompasses a group of inflammatory gastrointestinal diseases that are often relapsing and remitting in nature and include Ulcerative Colitis and Crohn’s Disease (CD). CD affects over half a million individuals in the US, and nearly 4.9 million individuals worldwide. Before the development of biologic agents for the treatment of CD, many patients struggled to achieve disease control leading to a high rate of morbidity and mortality. FDA approval of Infliximab in 1998 marked a revolutionary change in the management of CD, and biologics have since become a staple of CD treatment. Recent studies have demonstrated the benefit of early initiation of biologic therapy to prevent the progression of CD and associated complications. Upadacitinib is a JAK inhibitor approved in 2023 for treatment of CD and is effective in achieving remission. However, no studies evaluate the use of Upadacitinib in hospitalized CD patients. Our study aims to evaluate the efficacy of Upadacitinib in treating hospitalized CD patients as well as outcomes for these patients following discharge.
Methods: Our study is a retrospective chart review of 19 cases at the University of Michigan Hospital in which Upadacitinib was initiated for the treatment of CD in hospitalized patients over the age of 18. Patient disease activity was monitored following discharge. Primary endpoints include the need for surgical intervention, Upadacitinib discontinuation, and frequency of steroid-requiring flares.
Results: Among the 19 patients hospitalized for CD and initiated on Upadacitinib, 21% (4/19) required post-discharge surgery for disease control, 37% (7/19) had Upadacitinib discontinued due to uncontrolled disease, and 85% (6/7) of eligible patients did not require steroids for persistent symptoms at 90-day follow-up.
Discussion: Our initial results suggest favorable outcomes in hospitalized CD treated with Upadacitinib based on the primary endpoints. Our study is limited by the recency of Upadacitinib's approval for use in CD. More research is required to describe the specific population of CD patients that would most benefit from its use in-hospital. Specifically, further analyses should be performed to investigate differences in efficacy based on demographics, geography of CD, severity of CD, and prior or concurrent use of alternative biologic agents. Upadacitinib shows promise in treating hospitalized CD patients, potentially reducing the need for steroids and surgical interventions.
Disclosures:
Jawad Bouhamdan, MD1, Jeffrey A. Berinstein, MD2. P2610 - Efficacy and Outcomes of Upadacitinib (Rinvoq) Use in Hospitalized Crohn's Disease Patients, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
1University of Michigan Health, Ann Arbor, MI; 2University of Michigan, Ann Arbor, MI
Introduction: Inflammatory bowel disease encompasses a group of inflammatory gastrointestinal diseases that are often relapsing and remitting in nature and include Ulcerative Colitis and Crohn’s Disease (CD). CD affects over half a million individuals in the US, and nearly 4.9 million individuals worldwide. Before the development of biologic agents for the treatment of CD, many patients struggled to achieve disease control leading to a high rate of morbidity and mortality. FDA approval of Infliximab in 1998 marked a revolutionary change in the management of CD, and biologics have since become a staple of CD treatment. Recent studies have demonstrated the benefit of early initiation of biologic therapy to prevent the progression of CD and associated complications. Upadacitinib is a JAK inhibitor approved in 2023 for treatment of CD and is effective in achieving remission. However, no studies evaluate the use of Upadacitinib in hospitalized CD patients. Our study aims to evaluate the efficacy of Upadacitinib in treating hospitalized CD patients as well as outcomes for these patients following discharge.
Methods: Our study is a retrospective chart review of 19 cases at the University of Michigan Hospital in which Upadacitinib was initiated for the treatment of CD in hospitalized patients over the age of 18. Patient disease activity was monitored following discharge. Primary endpoints include the need for surgical intervention, Upadacitinib discontinuation, and frequency of steroid-requiring flares.
Results: Among the 19 patients hospitalized for CD and initiated on Upadacitinib, 21% (4/19) required post-discharge surgery for disease control, 37% (7/19) had Upadacitinib discontinued due to uncontrolled disease, and 85% (6/7) of eligible patients did not require steroids for persistent symptoms at 90-day follow-up.
Discussion: Our initial results suggest favorable outcomes in hospitalized CD treated with Upadacitinib based on the primary endpoints. Our study is limited by the recency of Upadacitinib's approval for use in CD. More research is required to describe the specific population of CD patients that would most benefit from its use in-hospital. Specifically, further analyses should be performed to investigate differences in efficacy based on demographics, geography of CD, severity of CD, and prior or concurrent use of alternative biologic agents. Upadacitinib shows promise in treating hospitalized CD patients, potentially reducing the need for steroids and surgical interventions.
Disclosures:
Jawad Bouhamdan indicated no relevant financial relationships.
Jeffrey Berinstein indicated no relevant financial relationships.
Jawad Bouhamdan, MD1, Jeffrey A. Berinstein, MD2. P2610 - Efficacy and Outcomes of Upadacitinib (Rinvoq) Use in Hospitalized Crohn's Disease Patients, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
