Monday Poster Session
Category: IBD
P2624 - Evaluating the Time Needed to Access Advanced Therapies for Crohn’s Disease and Ulcerative Colitis
Monday, October 28, 2024
10:30 AM - 4:00 PM ET
Location: Exhibit Hall E
- JC
J Casey Chapman, MD
GI Alliance
Baton Rouge, LA
Presenting Author(s)
Casey Chapman, MD1, Bincy P. Abraham, MD, MS, FACG2, Parakkal Deepak, MBBS, MS3, Tianzhou Yu, PhD, MS, MPH4, Jenny Griffith, PharmD5, Si Xuan, PhD6, Brooke Kim, RN, BSN, MS4, Cecile Holweg, PhD4, Jae Rok Kim, PharmD, MS7, Corey A.. Siegel, MD, MS8
1GI Alliance, Baton Rouge, LA; 2Houston Methodist-Weill Cornell, Houston, TX; 3Washington University in St. Louis, St. Louis, MO; 4AbbVie, Mettawa, IL; 5AbbVie, Edwardsville, IL; 6AbbVie, Lake County, IL; 7AbbVie, Irvine, CA; 8Dartmouth-Hitchcock Medical Center, Lebanon, NH
Introduction: Newly-approved advanced therapies in Crohn’s disease (CD) or ulcerative colitis (UC) may take longer for patients to access. Here, the time needed to access advanced therapy for patients with CD or UC was evaluated.
Methods: The Symphony Health Solutions administrative claims database was used to identify claims associated with the index treatment (biologic or small molecule oral medication) made between January 1, 2006, and December 31, 2023. Eligible patients for this retrospective cohort study were ≥18 years old at the index date (date of first claim after January 1, 2023), had ≥1 claims associated with a CD or UC diagnosis ≤6 months (mo) before the index date, had no claims associated with the index treatment before the index date, had ≥6mo of baseline medical/pharmacy coverage (≥1 medical and pharmacy claim ≤6mo before the index date) and ≥1 claim associated with gastroenterologist (GI) visit ≤3mo before the index date. The base case analysis used the last GI visit before the index date as the proxy for the prescription date of issue. Time to access (number of days [d] from the last GI visit to the index date for each drug-disease cohort) was assessed by disease (CD versus [vs] UC) and drug type (infusion vs self-administered) using analysis of variance. Subgroup analyses based on prior use of advanced therapy were also conducted. Sensitivity analysis compared the time to access self-administered drugs calculated in the base case and actual prescription written date.
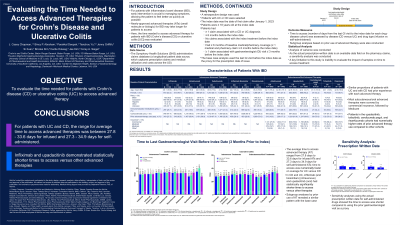
Results: The average time to access infused treatments ranged from 27.8d to 33.6d with infliximab (and biosimilars) in CD and UC, having significantly shorter times relative to other therapies (Table). The average time to access self-administered therapies ranged from 27.3d to 34.9d, with upadacitinib (UPA) having significantly shorter times relative to other therapies. Subgroup analyses by prior use of advanced therapy revealed a similar pattern with the base case. Sensitivity analyses using the actual prescription written date for self-administered drugs showed the time to access was shorter compared to using the prior GI visit as a proxy, with average time ranging from 9.0d with UPA (UC) to 30.5d for ozanimod.
Discussion: While there are significant differences in the time needed for patients with CD or UC to access advanced therapies, the differences are relatively small for infused therapies. A key limitation to this analysis is the inability to evaluate the impact of drug samples on access time.
Note: The table for this abstract can be viewed in the ePoster Gallery section of the ACG 2024 ePoster Site or in The American Journal of Gastroenterology's abstract supplement issue, both of which will be available starting October 27, 2024.
Disclosures:
Casey Chapman, MD1, Bincy P. Abraham, MD, MS, FACG2, Parakkal Deepak, MBBS, MS3, Tianzhou Yu, PhD, MS, MPH4, Jenny Griffith, PharmD5, Si Xuan, PhD6, Brooke Kim, RN, BSN, MS4, Cecile Holweg, PhD4, Jae Rok Kim, PharmD, MS7, Corey A.. Siegel, MD, MS8. P2624 - Evaluating the Time Needed to Access Advanced Therapies for Crohn’s Disease and Ulcerative Colitis, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
1GI Alliance, Baton Rouge, LA; 2Houston Methodist-Weill Cornell, Houston, TX; 3Washington University in St. Louis, St. Louis, MO; 4AbbVie, Mettawa, IL; 5AbbVie, Edwardsville, IL; 6AbbVie, Lake County, IL; 7AbbVie, Irvine, CA; 8Dartmouth-Hitchcock Medical Center, Lebanon, NH
Introduction: Newly-approved advanced therapies in Crohn’s disease (CD) or ulcerative colitis (UC) may take longer for patients to access. Here, the time needed to access advanced therapy for patients with CD or UC was evaluated.
Methods: The Symphony Health Solutions administrative claims database was used to identify claims associated with the index treatment (biologic or small molecule oral medication) made between January 1, 2006, and December 31, 2023. Eligible patients for this retrospective cohort study were ≥18 years old at the index date (date of first claim after January 1, 2023), had ≥1 claims associated with a CD or UC diagnosis ≤6 months (mo) before the index date, had no claims associated with the index treatment before the index date, had ≥6mo of baseline medical/pharmacy coverage (≥1 medical and pharmacy claim ≤6mo before the index date) and ≥1 claim associated with gastroenterologist (GI) visit ≤3mo before the index date. The base case analysis used the last GI visit before the index date as the proxy for the prescription date of issue. Time to access (number of days [d] from the last GI visit to the index date for each drug-disease cohort) was assessed by disease (CD versus [vs] UC) and drug type (infusion vs self-administered) using analysis of variance. Subgroup analyses based on prior use of advanced therapy were also conducted. Sensitivity analysis compared the time to access self-administered drugs calculated in the base case and actual prescription written date.
Results: The average time to access infused treatments ranged from 27.8d to 33.6d with infliximab (and biosimilars) in CD and UC, having significantly shorter times relative to other therapies (Table). The average time to access self-administered therapies ranged from 27.3d to 34.9d, with upadacitinib (UPA) having significantly shorter times relative to other therapies. Subgroup analyses by prior use of advanced therapy revealed a similar pattern with the base case. Sensitivity analyses using the actual prescription written date for self-administered drugs showed the time to access was shorter compared to using the prior GI visit as a proxy, with average time ranging from 9.0d with UPA (UC) to 30.5d for ozanimod.
Discussion: While there are significant differences in the time needed for patients with CD or UC to access advanced therapies, the differences are relatively small for infused therapies. A key limitation to this analysis is the inability to evaluate the impact of drug samples on access time.
Note: The table for this abstract can be viewed in the ePoster Gallery section of the ACG 2024 ePoster Site or in The American Journal of Gastroenterology's abstract supplement issue, both of which will be available starting October 27, 2024.
Disclosures:
Casey Chapman: AbbVie – Advisory Committee/Board Member, Consultant, Speakers Bureau. Bristol Myers Squibb – Speakers Bureau. El Lilly and Company – Consultant. Janssen – Speakers Bureau. Medtronic – Consultant. Pfizer – Advisory Committee/Board Member, Speakers Bureau. Takeda – Advisory Committee/Board Member, Speakers Bureau.
Bincy P. Abraham: AbbVie – Consultant, Speakers Bureau. Bristol Myers Squibb – Consultant, Speakers Bureau. Celltrion – Consultant. Eli Lilly – Consultant, Speakers Bureau. Fresenius Kabi – Consultant. Janssen – Consultant, Speakers Bureau. Medtronic – Consultant. Pfizer – Consultant, Speakers Bureau. Prometheus – Consultant. Samsung Bioepis – Consultant. Takeda – Consultant, Speakers Bureau.
Parakkal Deepak: AbbVie – Consultant, Grant/Research Support. Alimentiv – Grant/Research Support. Arena Pharmaceuticals – Grant/Research Support. Boehringer Ingelheim – Grant/Research Support. Bristol Myers Squibb-Celgene – Advisory Committee/Board Member, Grant/Research Support. CorEvitas LLC – Consultant. Janssen – Grant/Research Support. Pfizer – Grant/Research Support. Prometheus Biosciences – Grant/Research Support. Roche/Genentech – Advisory Committee/Board Member. Scipher Medicine – Grant/Research Support. Takeda – Grant/Research Support.
Tianzhou Yu: AbbVie – Fellowship sponsored by the company.
Jenny Griffith: AbbVie – Employee, Stock Options, Stock-publicly held company(excluding mutual/index funds).
Si Xuan indicated no relevant financial relationships.
Brooke Kim: AbbVie – Employee.
Cecile Holweg: AbbVie – Employee, Stock-publicly held company(excluding mutual/index funds).
Jae Rok Kim: AbbVie – Employee, Stock-publicly held company(excluding mutual/index funds).
Corey Siegel: Abbvie – Advisory Committee/Board Member, Consultant, Grant/Research Support, Speaker for CME activities. BMS – Advisory Committee/Board Member, Consultant. Boomerang – Advisory Committee/Board Member, Consultant. Buhlman – Advisory Committee/Board Member, Consultant. Janssen – Advisory Committee/Board Member, Consultant, Grant/Research Support, Speaker for CME activities. Lilly – Advisory Committee/Board Member, Consultant. Napo pharmaceuticals – Advisory Committee/Board Member, Consultant. Pfizer – Advisory Committee/Board Member, Consultant, Grant/Research Support, speaker for CME activities. Prometheus Biosciences – Advisory Committee/Board Member, Consultant. Prometheus Labs – Advisory Committee/Board Member, Consultant. Roivant – Advisory Committee/Board Member, Consultant. Takeda – Advisory Committee/Board Member, Consultant, Grant/Research Support, speaker for CME activities. Trellus Health – Advisory Committee/Board Member, Consultant.
Casey Chapman, MD1, Bincy P. Abraham, MD, MS, FACG2, Parakkal Deepak, MBBS, MS3, Tianzhou Yu, PhD, MS, MPH4, Jenny Griffith, PharmD5, Si Xuan, PhD6, Brooke Kim, RN, BSN, MS4, Cecile Holweg, PhD4, Jae Rok Kim, PharmD, MS7, Corey A.. Siegel, MD, MS8. P2624 - Evaluating the Time Needed to Access Advanced Therapies for Crohn’s Disease and Ulcerative Colitis, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
