Monday Poster Session
Category: IBD
P2547 - Identification of Moderate Disease Severity Definitions for Crohn’s Disease and Ulcerative Colitis: A Review of Phase 3 Trials of Pharmacological Therapy
Monday, October 28, 2024
10:30 AM - 4:00 PM ET
Location: Exhibit Hall E

- PD
Parambir S. Dulai, MD
Feinberg School of Medicine, Northwestern University
Chicago, IL
Presenting Author(s)
Chiahung Chou, PhD1, Marie Sanchirico, MD, PhD1, Richa Mukherjee, MSc, PhD1, David Hudesman, MD2, Timothy E. Ritter, MD3, Parambir S. Dulai, MD4
1Takeda Pharmaceuticals, USA, Inc., Cambridge, MA; 2NYU Langone Health Inflammatory Bowel Disease Center, New York, NY; 3GI Alliance, Southlake, TX; 4Feinberg School of Medicine, Northwestern University, Chicago, IL
Introduction: Distinguishing moderate from severe inflammatory bowel disease (IBD) is of interest because early treatment of IBD with biologics may improve clinical outcomes. We conducted a literature review to identify all definitions or stratifications of disease severity used in phase 3 trials of pharmacological therapy for Crohn’s disease (CD) or ulcerative colitis (UC).
Methods: PubMed and bibliographies of systematic literature reviews were searched on December 11, 2023 to identify primary publications of phase 3 trials from year 2000 onwards. Definitions and stratifications of disease severity, comprising the name of measurement and the score or range of scores used, were extracted from eligible publications.
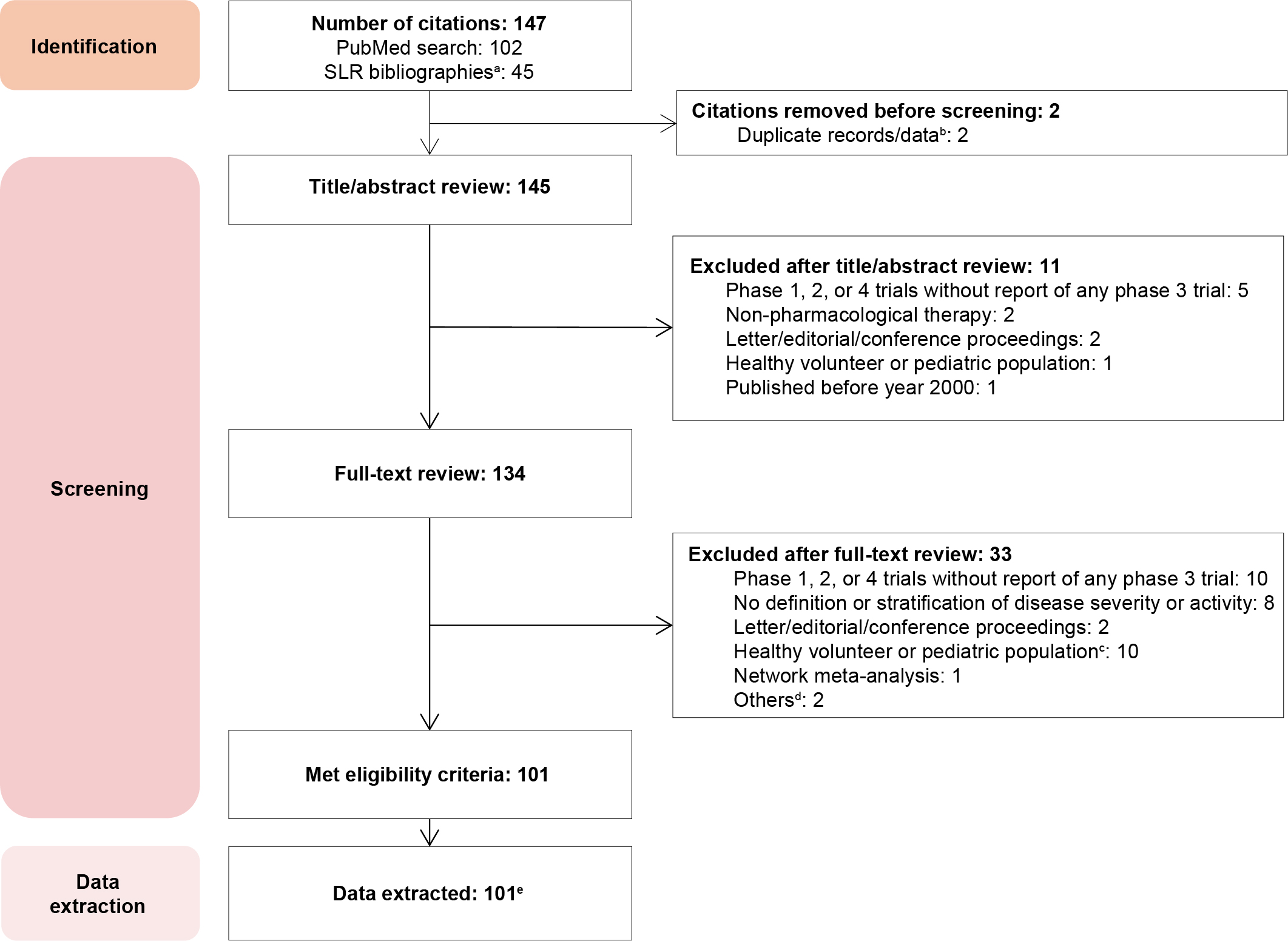
Results: Definitions were extracted from 101 publications (Figure 1). Most publications employed CD Activity Index (CDAI) scores to define mild-to-moderate (n = 2) and moderate-to-severe (n = 30) CD. The CDAI was often combined with other criteria, including those based on Simple Endoscopic Score (SES)-CD, C-reactive protein (CRP), and fecal calprotectin (FCP) to define moderate-to-severe disease. In UC, most publications used the modified Sutherland UC Disease Activity Index score and sub scores to define mild-to-moderate disease (N = 3) and the Mayo score and/or sub scores to define moderate-to-severe (N = 26) disease. The definitions of moderate CD or UC were few and inconsistent; all were used to stratify patients with mild-to-moderate or moderate-to-severe disease (Table 1). No biomarkers were used to define moderate CD or UC; however, FCP and CRP levels were used to stratify patients with moderate-to-severe disease into cohorts using cut-offs that varied among publications.
Discussion: In this literature review, no consensus definition of moderate CD or UC was identified in publications of phase 3 trials of pharmacological therapy. The definitions of moderate CD and UC were inconsistent and relied on measures of disease activity, which have limited utility in clinical practice. Developing a clinically relevant definition of moderate disease that encompasses clinical and endoscopic measures, biomarker levels, and disease prognosis or trajectory is important to improve long-term outcomes of IBD.
Note: The table for this abstract can be viewed in the ePoster Gallery section of the ACG 2024 ePoster Site or in The American Journal of Gastroenterology's abstract supplement issue, both of which will be available starting October 27, 2024.
Disclosures:
Chiahung Chou, PhD1, Marie Sanchirico, MD, PhD1, Richa Mukherjee, MSc, PhD1, David Hudesman, MD2, Timothy E. Ritter, MD3, Parambir S. Dulai, MD4. P2547 - Identification of Moderate Disease Severity Definitions for Crohn’s Disease and Ulcerative Colitis: A Review of Phase 3 Trials of Pharmacological Therapy, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
1Takeda Pharmaceuticals, USA, Inc., Cambridge, MA; 2NYU Langone Health Inflammatory Bowel Disease Center, New York, NY; 3GI Alliance, Southlake, TX; 4Feinberg School of Medicine, Northwestern University, Chicago, IL
Introduction: Distinguishing moderate from severe inflammatory bowel disease (IBD) is of interest because early treatment of IBD with biologics may improve clinical outcomes. We conducted a literature review to identify all definitions or stratifications of disease severity used in phase 3 trials of pharmacological therapy for Crohn’s disease (CD) or ulcerative colitis (UC).
Methods: PubMed and bibliographies of systematic literature reviews were searched on December 11, 2023 to identify primary publications of phase 3 trials from year 2000 onwards. Definitions and stratifications of disease severity, comprising the name of measurement and the score or range of scores used, were extracted from eligible publications.
Results: Definitions were extracted from 101 publications (Figure 1). Most publications employed CD Activity Index (CDAI) scores to define mild-to-moderate (n = 2) and moderate-to-severe (n = 30) CD. The CDAI was often combined with other criteria, including those based on Simple Endoscopic Score (SES)-CD, C-reactive protein (CRP), and fecal calprotectin (FCP) to define moderate-to-severe disease. In UC, most publications used the modified Sutherland UC Disease Activity Index score and sub scores to define mild-to-moderate disease (N = 3) and the Mayo score and/or sub scores to define moderate-to-severe (N = 26) disease. The definitions of moderate CD or UC were few and inconsistent; all were used to stratify patients with mild-to-moderate or moderate-to-severe disease (Table 1). No biomarkers were used to define moderate CD or UC; however, FCP and CRP levels were used to stratify patients with moderate-to-severe disease into cohorts using cut-offs that varied among publications.
Discussion: In this literature review, no consensus definition of moderate CD or UC was identified in publications of phase 3 trials of pharmacological therapy. The definitions of moderate CD and UC were inconsistent and relied on measures of disease activity, which have limited utility in clinical practice. Developing a clinically relevant definition of moderate disease that encompasses clinical and endoscopic measures, biomarker levels, and disease prognosis or trajectory is important to improve long-term outcomes of IBD.

Figure: Figure 1. Flow-chart of publication counts in the literature review.
CD, Crohn’s disease; SLR, systemic literature review; UC, ulcerative colitis.
a The SLRs were conducted as part of network meta-analyses (unpublished). b Duplicates were identified and removed manually. c Trial populations with patients who were less than 18 years of age. d The full-text of one publication was unavailable, and the trial phase was not mentioned in the other publication. e 47 publications related to CD only, 49 to UC only, and 5 to both CD and UC.
CD, Crohn’s disease; SLR, systemic literature review; UC, ulcerative colitis.
a The SLRs were conducted as part of network meta-analyses (unpublished). b Duplicates were identified and removed manually. c Trial populations with patients who were less than 18 years of age. d The full-text of one publication was unavailable, and the trial phase was not mentioned in the other publication. e 47 publications related to CD only, 49 to UC only, and 5 to both CD and UC.
Note: The table for this abstract can be viewed in the ePoster Gallery section of the ACG 2024 ePoster Site or in The American Journal of Gastroenterology's abstract supplement issue, both of which will be available starting October 27, 2024.
Disclosures:
Chiahung Chou: Takeda Pharmaceuticals U.S.A., Inc. – Employee, Stock Options.
Marie Sanchirico: Takeda Pharmaceuticals U.S.A., Inc. – Employee, Stock Options.
Richa Mukherjee: Takeda Pharmaceuticals U.S.A., Inc. – Employee, Stock Options.
David Hudesman: Abbvie – Consultant, Grant/Research Support. Avalo – Consultant. BMS – Consultant. Eli Lilly – Consultant. Fresenius Kabi – Consultant. Johnson and Johnson – Consultant. Pfizer – Consultant, Grant/Research Support. Prometheus – Consultant. Takeda – Consultant.
Timothy Ritter: AbbVie – Advisory Committee/Board Member, Speakers Bureau. Ardelyx – Advisory Committee/Board Member. Arena Pharmaceuticals – Advisory Committee/Board Member. Boehringer Ingelheim – Advisory Committee/Board Member. Bristol Myers Squibb – Advisory Committee/Board Member, Speakers Bureau. Celgene – Advisory Committee/Board Member. Eli Lilly – Advisory Committee/Board Member, Speakers Bureau. Ferring – Advisory Committee/Board Member. Ferring/Rebiotix – has served on a Data Adjudication Committee. Genentech (Roche) – Advisory Committee/Board Member. Intercept – Advisory Committee/Board Member. Iterative Scopes – Advisory Committee/Board Member, holds shares in Iterative Scopes. Janssen – Advisory Committee/Board Member, Speakers Bureau. Nestlé/Seres – Advisory Committee/Board Member. Pfizer – Advisory Committee/Board Member, Speakers Bureau. Prometheus – Advisory Committee/Board Member. Sanofi – Advisory Committee/Board Member. Takeda – Advisory Committee/Board Member, Speakers Bureau.
Parambir Dulai: AbbVie – Consultant. Abivax – Consultant. Adiso – Consultant. Bristol Meyer Squibb – Consultant. Digbi Health – Royalties. Digbi Health – Stock Options. Geneoscopy – Consultant. GSK – Consultant. Janssen – Consultant. Lilly – Consultant. Pfizer – Consultant, Grant/Research Support. Precidiag – Licensing royalties. Takeda – Consultant, Grant/Research Support.
Chiahung Chou, PhD1, Marie Sanchirico, MD, PhD1, Richa Mukherjee, MSc, PhD1, David Hudesman, MD2, Timothy E. Ritter, MD3, Parambir S. Dulai, MD4. P2547 - Identification of Moderate Disease Severity Definitions for Crohn’s Disease and Ulcerative Colitis: A Review of Phase 3 Trials of Pharmacological Therapy, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
