Monday Poster Session
Category: IBD
P2548 - Real-World Evidence on the Effectiveness and Safety of Tofacitinib in Patients With Ulcerative Colitis in Lebanon
Monday, October 28, 2024
10:30 AM - 4:00 PM ET
Location: Exhibit Hall E
Has Audio
- AS
Ala I. Sharara, MD, FACG
American University of Beirut
Beyrouth, Beyrouth, Lebanon
Presenting Author(s)
Ala I. Sharara, MD, FACG1, Ayman Alrazim, MD2, Philippe Saniour, MD3, Fady Daniel, MD1, Antoine Abou Rached, MD4, Abbas Bahr, MD5, Cecilio Azar, MD1, Antoine Geagea, MD6, Marcelle Ghoubar, PharmD7
1American University of Beirut, Beyrouth, Beyrouth, Lebanon; 2American University of Beirut, Beirut, Beyrouth, Lebanon; 3St. Joseph Hospital, Beirut, Beyrouth, Lebanon; 4Lebanese American University School of Medicine, Beirut, Beyrouth, Lebanon; 5Bahman Hospital, Beirut, Beyrouth, Lebanon; 6Levant Hospital, Beirut, Beyrouth, Lebanon; 7Pfizer Inc., Beirut, Beyrouth, Lebanon
Introduction: To evaluate the effectiveness and safety of tofacitinib in patients with ulcerative colitis (UC) in clinical practice in Lebanon.
Methods: This was a retrospective cross-sectional study. Patients with moderate to severe UC treated with tofacitinib between 2018 and 2021 were included. Patients’ demographics, disease-specific characteristics, clinical assessment at three time points (8, 26, and 52 weeks), endoscopic evaluation at 24 weeks, and adverse events were collected.
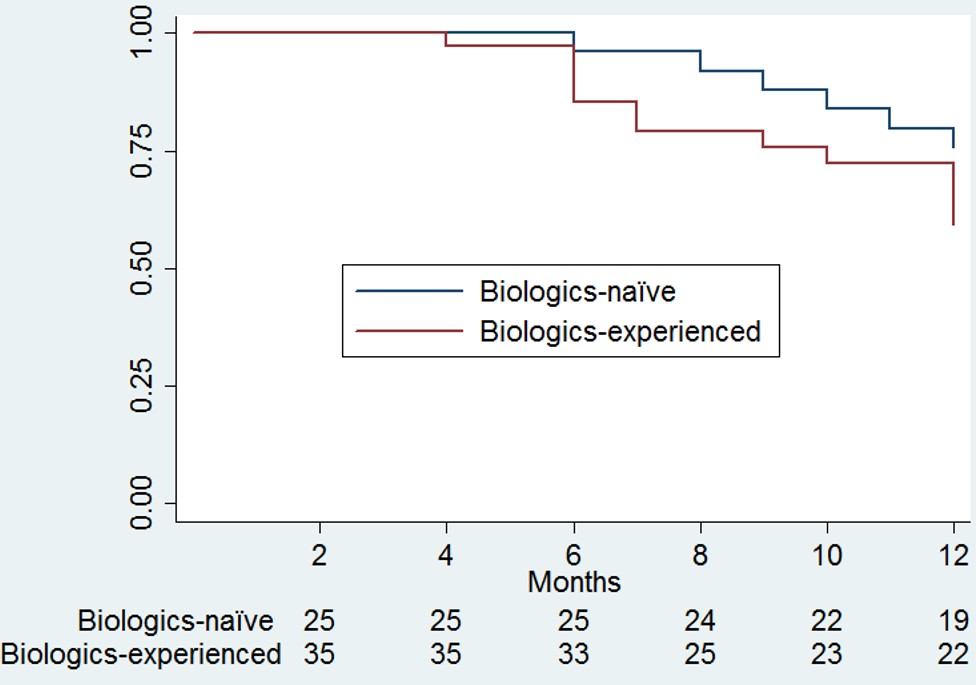
Results: A total of 60 UC patients with a mean (SD) duration of disease of 7.9 ± SD 4.7 years were enrolled. 61.7% of patients had extensive disease, and 58.3% had received ≥ 1 biologic prior to tofacitinib. Clinical remission was reported in 25, 34, and 31 patients (41.7%, 56.7%, and 56.4%) at 8, 26, and 52 weeks respectively (Figure 1I). Endoscopic remission (endoscopic Mayo score 0 or 1) was observed in 58.3% of patients. About one-third of patients (31.7%) stopped tofacitinib at one year, primarily for lack of efficacy or loss of response, with no significant difference between biologics-naïve and experienced patients (24% vs. 37.1% respectively; Figure 2). No serious adverse events or deaths were reported. Adverse events were reported in 3 patients (5.0%) - one C. difficile infection, one case of reversible lymphopenia, and one case of facial acne. No serious adverse events or deaths were noted. On multivariate analysis, biologic-naïve status and reduction or normalization of CRP were associated with clinical remission (OR = 10.87 [95% CI =1.57, 100] and OR = 78.47 [95% CI =2.09, 2940.32], respectively), while reduction or normalization of CRP was associated with endoscopic remission at 1 year (OR = 19.03 [95% CI =1.64, 221.09]).
Discussion: Tofacitinib was effective in the treatment of moderately severe ulcerative colitis in this real-world cohort in Lebanon. Further, the predictors associated with clinical and endoscopic remissions were found to be biologic-naïve status and reduction in CRP. Observed AEs were consistent with the known safety profile. One of the major limitations of this study is the smaller sample size and the retrospective nature of the study.
Note: The table for this abstract can be viewed in the ePoster Gallery section of the ACG 2024 ePoster Site or in The American Journal of Gastroenterology's abstract supplement issue, both of which will be available starting October 27, 2024.
Disclosures:
Ala I. Sharara, MD, FACG1, Ayman Alrazim, MD2, Philippe Saniour, MD3, Fady Daniel, MD1, Antoine Abou Rached, MD4, Abbas Bahr, MD5, Cecilio Azar, MD1, Antoine Geagea, MD6, Marcelle Ghoubar, PharmD7. P2548 - Real-World Evidence on the Effectiveness and Safety of Tofacitinib in Patients With Ulcerative Colitis in Lebanon, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
1American University of Beirut, Beyrouth, Beyrouth, Lebanon; 2American University of Beirut, Beirut, Beyrouth, Lebanon; 3St. Joseph Hospital, Beirut, Beyrouth, Lebanon; 4Lebanese American University School of Medicine, Beirut, Beyrouth, Lebanon; 5Bahman Hospital, Beirut, Beyrouth, Lebanon; 6Levant Hospital, Beirut, Beyrouth, Lebanon; 7Pfizer Inc., Beirut, Beyrouth, Lebanon
Introduction: To evaluate the effectiveness and safety of tofacitinib in patients with ulcerative colitis (UC) in clinical practice in Lebanon.
Methods: This was a retrospective cross-sectional study. Patients with moderate to severe UC treated with tofacitinib between 2018 and 2021 were included. Patients’ demographics, disease-specific characteristics, clinical assessment at three time points (8, 26, and 52 weeks), endoscopic evaluation at 24 weeks, and adverse events were collected.
Results: A total of 60 UC patients with a mean (SD) duration of disease of 7.9 ± SD 4.7 years were enrolled. 61.7% of patients had extensive disease, and 58.3% had received ≥ 1 biologic prior to tofacitinib. Clinical remission was reported in 25, 34, and 31 patients (41.7%, 56.7%, and 56.4%) at 8, 26, and 52 weeks respectively (Figure 1I). Endoscopic remission (endoscopic Mayo score 0 or 1) was observed in 58.3% of patients. About one-third of patients (31.7%) stopped tofacitinib at one year, primarily for lack of efficacy or loss of response, with no significant difference between biologics-naïve and experienced patients (24% vs. 37.1% respectively; Figure 2). No serious adverse events or deaths were reported. Adverse events were reported in 3 patients (5.0%) - one C. difficile infection, one case of reversible lymphopenia, and one case of facial acne. No serious adverse events or deaths were noted. On multivariate analysis, biologic-naïve status and reduction or normalization of CRP were associated with clinical remission (OR = 10.87 [95% CI =1.57, 100] and OR = 78.47 [95% CI =2.09, 2940.32], respectively), while reduction or normalization of CRP was associated with endoscopic remission at 1 year (OR = 19.03 [95% CI =1.64, 221.09]).
Discussion: Tofacitinib was effective in the treatment of moderately severe ulcerative colitis in this real-world cohort in Lebanon. Further, the predictors associated with clinical and endoscopic remissions were found to be biologic-naïve status and reduction in CRP. Observed AEs were consistent with the known safety profile. One of the major limitations of this study is the smaller sample size and the retrospective nature of the study.

Figure: Persistence on Tofacitinib in the study cohort at one year stratified by biologics-exposure
Note: The table for this abstract can be viewed in the ePoster Gallery section of the ACG 2024 ePoster Site or in The American Journal of Gastroenterology's abstract supplement issue, both of which will be available starting October 27, 2024.
Disclosures:
Ala Sharara indicated no relevant financial relationships.
Ayman Alrazim indicated no relevant financial relationships.
Philippe Saniour indicated no relevant financial relationships.
Fady Daniel indicated no relevant financial relationships.
Antoine Abou Rached indicated no relevant financial relationships.
Abbas Bahr indicated no relevant financial relationships.
Cecilio Azar indicated no relevant financial relationships.
Antoine Geagea indicated no relevant financial relationships.
Marcelle Ghoubar indicated no relevant financial relationships.
Ala I. Sharara, MD, FACG1, Ayman Alrazim, MD2, Philippe Saniour, MD3, Fady Daniel, MD1, Antoine Abou Rached, MD4, Abbas Bahr, MD5, Cecilio Azar, MD1, Antoine Geagea, MD6, Marcelle Ghoubar, PharmD7. P2548 - Real-World Evidence on the Effectiveness and Safety of Tofacitinib in Patients With Ulcerative Colitis in Lebanon, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
