Monday Poster Session
Category: IBD
P2558 - A Survey Among IBD Patients Following Switching From Originator Infliximab to Its Biosimilars
Monday, October 28, 2024
10:30 AM - 4:00 PM ET
Location: Exhibit Hall E

Has Audio
- AD
Anh Trinh Doan, DO
Baylor Scott & White Medical Center
Belton, TX
Presenting Author(s)
Anh Trinh Doan, DO1, Christopher Johnson, MD2
1Baylor Scott & White Medical Center, Belton, TX; 2Baylor Scott & White Medical Center, Temple, TX
Introduction: The biologic agent -Infliximab (IFX) was first introduced two decades ago and revolutionized the treatment for Inflammatory bowel disease (IBD), comprised of Ulcerative Colitis (UC) and Crohn's disease (CD). Since 2016, the FDA has approved three IFX biosimilar agents (SB2), Renflexis®, Avsola™, and Inflectra®. Many IBD patients who achieve remission with one form of IFX must be switched to biosimilars due to their cost-savings. Numerous studies have proven their efficacy compared to IFX. However, few focused on real-world patients' experiences, especially in the United States.
Methods: We enrolled IBD patients aged 18 and older who were treated with IFX between January 1, 2019, and January 1, 2023 at an IBD center- Baylor Scott and White (BSW), Temple, Texas. Through chart-check, we select patients in clinical remission from Remicade for at least three months before switching to an SB2. We then conducted a satisfactory survey by calling the selected patients and asking them to rate their experience with SB2 though three questions using the Linkert scale.
Q1: How well did your provider explain the medication changes before starting it?
Q2: How did you feel about your symptoms control compared to before?
Q3: Overall experience with biosimilar medicine.
Results:
Discussion: We got an overwhelmingly positive response from patients regarding their satisfaction with the SB2. Most patients received good counseling about the medicine switch and thought SB2 worked the same way as the originator. They liked SB2 due to its cost-saving properties. This further crystallized our hypothesis of equivalent efficacy between the IFX originator and SB2. We also identified the patient’s biggest complaints about insurance and copay. They would like providers to integrate costs into counseling sessions.
Disclosures:
Anh Trinh Doan, DO1, Christopher Johnson, MD2. P2558 - A Survey Among IBD Patients Following Switching From Originator Infliximab to Its Biosimilars, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
1Baylor Scott & White Medical Center, Belton, TX; 2Baylor Scott & White Medical Center, Temple, TX
Introduction: The biologic agent -Infliximab (IFX) was first introduced two decades ago and revolutionized the treatment for Inflammatory bowel disease (IBD), comprised of Ulcerative Colitis (UC) and Crohn's disease (CD). Since 2016, the FDA has approved three IFX biosimilar agents (SB2), Renflexis®, Avsola™, and Inflectra®. Many IBD patients who achieve remission with one form of IFX must be switched to biosimilars due to their cost-savings. Numerous studies have proven their efficacy compared to IFX. However, few focused on real-world patients' experiences, especially in the United States.
Methods: We enrolled IBD patients aged 18 and older who were treated with IFX between January 1, 2019, and January 1, 2023 at an IBD center- Baylor Scott and White (BSW), Temple, Texas. Through chart-check, we select patients in clinical remission from Remicade for at least three months before switching to an SB2. We then conducted a satisfactory survey by calling the selected patients and asking them to rate their experience with SB2 though three questions using the Linkert scale.
Q1: How well did your provider explain the medication changes before starting it?
Q2: How did you feel about your symptoms control compared to before?
Q3: Overall experience with biosimilar medicine.
Results:
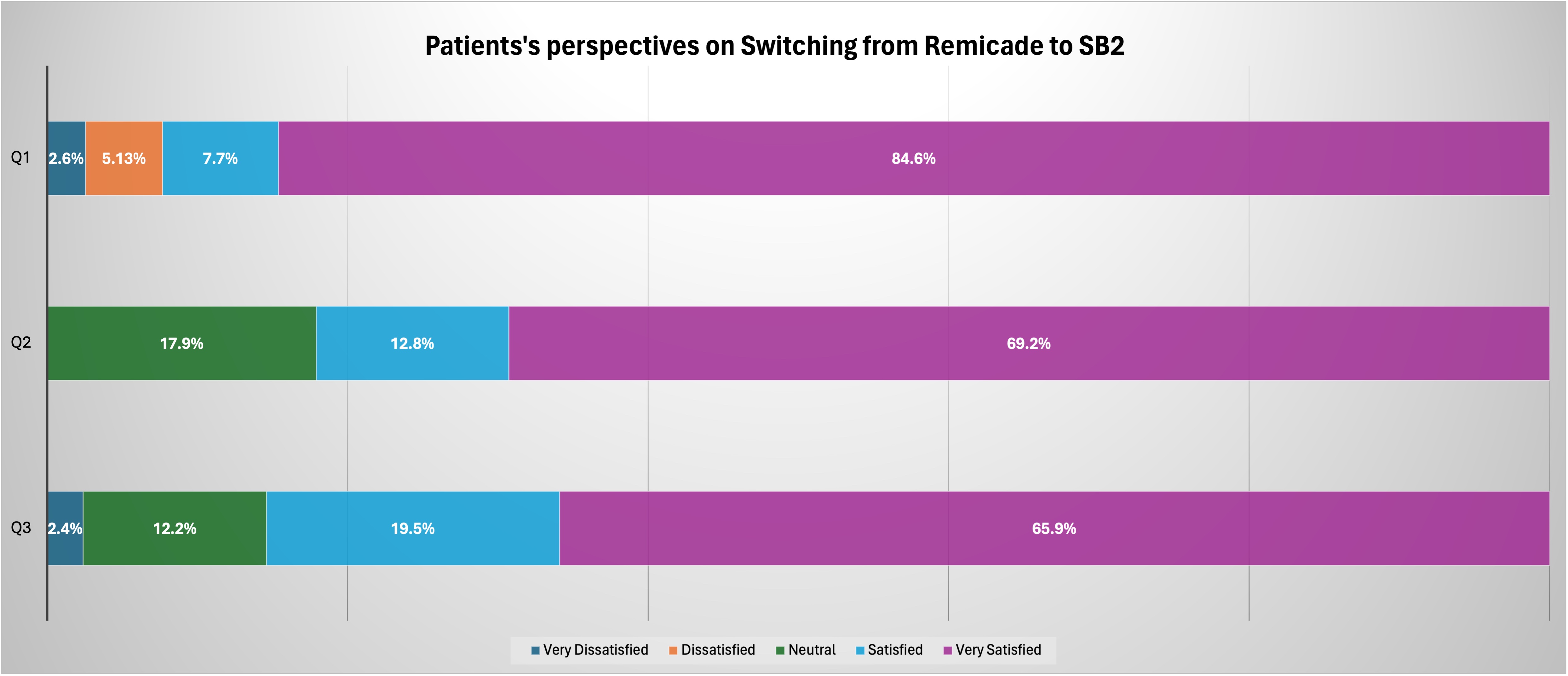
Sixty-seven eligible patients (12 UC and 55 CD) were enrolled in the study, with 41 patients completing the survey. For question 1 (Q1), 84.6% of respondents answered “very satisfied,” indicating that their providers did an excellent job counseling them on SB2. Regarding symptom control (Q2) and overall experience with SB2 (Q3), 82% and 85.4% of patients, respectively, rated their satisfaction as “Satisfied” or above. We received six negative reviews, four of which were due to financial reasons. Out of the 11 patients who met the primary outcome for failed therapy within the first year, we successfully interviewed five. Interestingly, all five respondents reported high satisfaction with all three questions.
Discussion: We got an overwhelmingly positive response from patients regarding their satisfaction with the SB2. Most patients received good counseling about the medicine switch and thought SB2 worked the same way as the originator. They liked SB2 due to its cost-saving properties. This further crystallized our hypothesis of equivalent efficacy between the IFX originator and SB2. We also identified the patient’s biggest complaints about insurance and copay. They would like providers to integrate costs into counseling sessions.

Figure: Table 1: Satisfactory survey response:
Q1: How well did your provider explain the medication changes before starting it
Q2: How did you feel about your symptoms control compared to before
Q3: Overall experience with biosimilar medicine.
Q1: How well did your provider explain the medication changes before starting it
Q2: How did you feel about your symptoms control compared to before
Q3: Overall experience with biosimilar medicine.
Disclosures:
Anh Trinh Doan indicated no relevant financial relationships.
Christopher Johnson indicated no relevant financial relationships.
Anh Trinh Doan, DO1, Christopher Johnson, MD2. P2558 - A Survey Among IBD Patients Following Switching From Originator Infliximab to Its Biosimilars, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
