Monday Poster Session
Category: IBD
P2559 - A Novel Remote Patient and Medication Monitoring Solution to Improve Adherence and PerSiStence With Inflammatory Bowel DiSease Therapy: Updates from the ASSIST Study
Monday, October 28, 2024
10:30 AM - 4:00 PM ET
Location: Exhibit Hall E
Has Audio

Jordan Axelrad, MD, MPH
NYU Grossman School of Medicine
New York, NY
Presenting Author(s)
Jordan Axelrad, MD, MPH1, Tamar Sapir, PhD2, Sara N. Horst, MD3, Kara DeFelice, MD4, Anita Afzali, MD, MPH, MHCM, FACG4, Aya Abdelrehem, BS1, Abigail Noyes, BS5, Chelsea Anderson, PhD, MPH6, Millie D. Long, MD, MPH7, Raymond K. Cross, MD, MS, FACG8
1NYU Grossman School of Medicine, New York, NY; 2Synchronyx, Boca Raton, FL; 3Vanderbilt Inflammatory Bowel Disease Clinic, Nashville, TN; 4University of Cincinnati College of Medicine, Cincinnati, OH; 5University of Maryland School of Medicine, Baltimore, MD; 6University of North Carolina, Chapel Hill, NC; 7Center for Gastrointestinal Biology and Disease, University of North Carolina, Chapel Hill, NC; 8Melissa L. Posner Institute for Digestive Health & Liver Disease at Mercy Medical Center, Baltimore, MD
Introduction: Suboptimal adherence to medical therapies for inflammatory bowel disease (IBD) is common and associated with adverse outcomes. Remote platforms have been developed to monitor adherence and guide interventions. In this study, we randomized patients with IBD initiating a medical therapy to a novel monitoring technology, Tappt, to improve adherence and clinical outcomes.
Methods: In an ongoing 12-month, multicenter study, patients with ulcerative colitis (UC) or Crohn’s disease (CD) initiating a new oral or subcutaneous treatment from 5 tertiary referral centers were randomized 2:1 to the Tappt web-based remote monitoring system or standard of care, respectively. The Tappt system utilized smart labels affixed to the pill bottle, pen, syringe, or an infusion card if receiving intravenous induction dosing. At the time of a dose, participants scanned the label by tapping it with their mobile device. The Tappt system captured self-reported adherence barriers and patient report outcomes (PROs). Email alerts were sent to the clinical team if a participant’s adherence or PROs fell below predetermined thresholds.
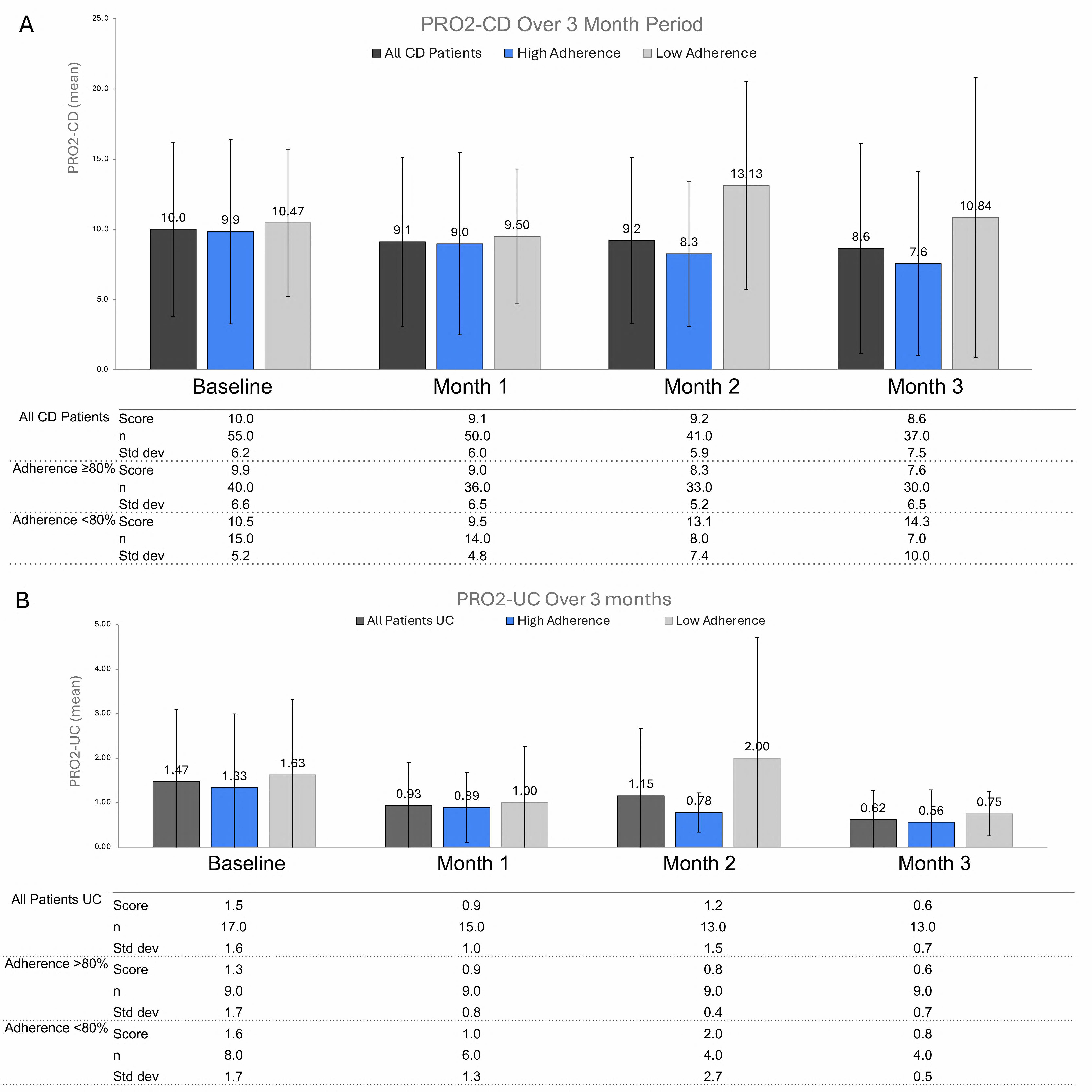
Results: Thus far, we enrolled 128 patients with IBD (77% CD, 23% UC). The mean age at enrollment was 36+/-12 years, and the majority were female (62%), white (83%), and had private insurance (87%; Table 1). Overall, 87 were randomized to the Tappt system and 41 to standard care. At baseline, indices of disease activity were similar across groups with 17% on concomitant corticosteroids. Medications initiated included mesalamine (2%), adalimumab (5%), ustekinumab (22%), upadacitinib (24%), and risankizumab (47%). Thus far, 16 completed the entire study and 26 withdrew. Focusing on those randomized to Tappt, median baseline Harvey Bradshaw and Simple Clinical Colitis Activity indices were 4 (IQR 2-6) and 4.5 (IQR 3-7) for patients with CD and UC, respectively. Among the Tappt group, adherence was 85%; 93% for ustekinumab, 89% for risankizumab, 81% for upadacitinib, 68% for adalimumab, and 32% for mesalamine. On average, 75% completed monthly PRO assessments. Over the first 3 months of follow up, there was a greater proportion who reported improvement in PRO2s among those with high adherence ( >80%, Figure). Most patients reported that Tappt was easy to use and were aided by medication reminders.
Discussion: In this ongoing, multicenter study of a novel remote monitoring technology, medication adherence was high, which was associated with improvement in PROs.
Note: The table for this abstract can be viewed in the ePoster Gallery section of the ACG 2024 ePoster Site or in The American Journal of Gastroenterology's abstract supplement issue, both of which will be available starting October 27, 2024.
Disclosures:
Jordan Axelrad, MD, MPH1, Tamar Sapir, PhD2, Sara N. Horst, MD3, Kara DeFelice, MD4, Anita Afzali, MD, MPH, MHCM, FACG4, Aya Abdelrehem, BS1, Abigail Noyes, BS5, Chelsea Anderson, PhD, MPH6, Millie D. Long, MD, MPH7, Raymond K. Cross, MD, MS, FACG8. P2559 - A Novel Remote Patient and Medication Monitoring Solution to Improve Adherence and PerSiStence With Inflammatory Bowel DiSease Therapy: Updates from the ASSIST Study, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
1NYU Grossman School of Medicine, New York, NY; 2Synchronyx, Boca Raton, FL; 3Vanderbilt Inflammatory Bowel Disease Clinic, Nashville, TN; 4University of Cincinnati College of Medicine, Cincinnati, OH; 5University of Maryland School of Medicine, Baltimore, MD; 6University of North Carolina, Chapel Hill, NC; 7Center for Gastrointestinal Biology and Disease, University of North Carolina, Chapel Hill, NC; 8Melissa L. Posner Institute for Digestive Health & Liver Disease at Mercy Medical Center, Baltimore, MD
Introduction: Suboptimal adherence to medical therapies for inflammatory bowel disease (IBD) is common and associated with adverse outcomes. Remote platforms have been developed to monitor adherence and guide interventions. In this study, we randomized patients with IBD initiating a medical therapy to a novel monitoring technology, Tappt, to improve adherence and clinical outcomes.
Methods: In an ongoing 12-month, multicenter study, patients with ulcerative colitis (UC) or Crohn’s disease (CD) initiating a new oral or subcutaneous treatment from 5 tertiary referral centers were randomized 2:1 to the Tappt web-based remote monitoring system or standard of care, respectively. The Tappt system utilized smart labels affixed to the pill bottle, pen, syringe, or an infusion card if receiving intravenous induction dosing. At the time of a dose, participants scanned the label by tapping it with their mobile device. The Tappt system captured self-reported adherence barriers and patient report outcomes (PROs). Email alerts were sent to the clinical team if a participant’s adherence or PROs fell below predetermined thresholds.
Results: Thus far, we enrolled 128 patients with IBD (77% CD, 23% UC). The mean age at enrollment was 36+/-12 years, and the majority were female (62%), white (83%), and had private insurance (87%; Table 1). Overall, 87 were randomized to the Tappt system and 41 to standard care. At baseline, indices of disease activity were similar across groups with 17% on concomitant corticosteroids. Medications initiated included mesalamine (2%), adalimumab (5%), ustekinumab (22%), upadacitinib (24%), and risankizumab (47%). Thus far, 16 completed the entire study and 26 withdrew. Focusing on those randomized to Tappt, median baseline Harvey Bradshaw and Simple Clinical Colitis Activity indices were 4 (IQR 2-6) and 4.5 (IQR 3-7) for patients with CD and UC, respectively. Among the Tappt group, adherence was 85%; 93% for ustekinumab, 89% for risankizumab, 81% for upadacitinib, 68% for adalimumab, and 32% for mesalamine. On average, 75% completed monthly PRO assessments. Over the first 3 months of follow up, there was a greater proportion who reported improvement in PRO2s among those with high adherence ( >80%, Figure). Most patients reported that Tappt was easy to use and were aided by medication reminders.
Discussion: In this ongoing, multicenter study of a novel remote monitoring technology, medication adherence was high, which was associated with improvement in PROs.

Figure: Monthly PRO2s among those with high adherence (>80%) and low adherence (<80%) in those with CD (A) and UC (B).
Note: The table for this abstract can be viewed in the ePoster Gallery section of the ACG 2024 ePoster Site or in The American Journal of Gastroenterology's abstract supplement issue, both of which will be available starting October 27, 2024.
Disclosures:
Jordan Axelrad: Abbvie – Consultant. Adiso – Consultant. Biomerieux – Consultant, Grant/Research Support. BMS – Consultant. Fresenius – Consultant. Genentech – Grant/Research Support. Janssen – Consultant. Pfizer – Consultant.
Tamar Sapir: Synchronyx – Employee, Owner/Ownership Interest.
Sara Horst: AbbVie – Consultant. Bristol Myers Squibb – Consultant. Janssen – Consultant. Pfizer – Consultant. Takeda – Consultant.
Kara DeFelice: Abbvie – Advisory Committee/Board Member, Speakers Bureau. BMS – Advisory Committee/Board Member, Speakers Bureau. Janssen – Honorary Speaker.
Anita Afzali: AbbVie – Consultant. Bristol Myers Squibb/Celgene – Consultant. Cullgen – Consultant. DiaSorin – Consultant. Eli Lilly – Consultant. Gilead – Consultant. IBD Horizons – Consultant. Janssen – Consultant. Pfizer – Consultant. Takeda – Consultant. TLL Pharmaceuticals – Consultant.
Aya Abdelrehem indicated no relevant financial relationships.
Abigail Noyes indicated no relevant financial relationships.
Chelsea Anderson indicated no relevant financial relationships.
Millie Long: AbbVie – Consultant. Bristol-Myers Squibb – Consultant. Eli Lilly – Consultant, Grant/Research Support. Intercept – Consultant. Janssen – Consultant. Pfizer Inc – Consultant, Grant/Research Support. Prometheus – Consultant. Roche – Consultant. Roivant – Consultant. Takeda – Consultant, Grant/Research Support. Target RWE – Consultant.
Raymond Cross: AbbVie – Consultant. Adiso – Consultant. Bristol Myers Squibb – Consultant. CorEvitas Registry – Scientific co-director. Fresenius Kabi – Consultant. Fzata – Consultant. IBD Education Group – Executive committee member. Janssen – Consultant, Grant/Research Support. Magellan Health – Consultant. Option Care Health – Consultant. Pfizer – Consultant. Pharmacosmos – Consultant. Samsung Bioepis – Consultant. Sandoz – Consultant. Sebela – Consultant. Takeda – Consultant.
Jordan Axelrad, MD, MPH1, Tamar Sapir, PhD2, Sara N. Horst, MD3, Kara DeFelice, MD4, Anita Afzali, MD, MPH, MHCM, FACG4, Aya Abdelrehem, BS1, Abigail Noyes, BS5, Chelsea Anderson, PhD, MPH6, Millie D. Long, MD, MPH7, Raymond K. Cross, MD, MS, FACG8. P2559 - A Novel Remote Patient and Medication Monitoring Solution to Improve Adherence and PerSiStence With Inflammatory Bowel DiSease Therapy: Updates from the ASSIST Study, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
