Monday Poster Session
Category: IBD
P2563 - Efficacy of Dose Escalation of Ustekinumab in Inflammatory Bowel Disease: A Systematic Review and Meta-Analysis
Monday, October 28, 2024
10:30 AM - 4:00 PM ET
Location: Exhibit Hall E

Has Audio

Nouman Shafique, MD
AdventHealth Medical Group, AdventHealth
Orlando, FL
Presenting Author(s)
Mohammad Ebad Ur Rehman, MBBS1, Abdul Qadeer Khan, MBBS2, Maha Sajjad, MBBS3, Ammara Tahir, MBBS4, Abu Huraira Bin Gulzar, 5, Aizaz Ali, MBBS6, Amna Hussain, MBBS7, Fatimah Shahid, MBBS1, Shahroon Zahid, MBBS8, Talha Bin. Yasin, MBBS9, Aqsa Bilal, MBBS10, Umara Rajput, MBBS11, Tehreem Fatima, MBBS12, Tehseen Haider, MBBS1, Sajeel Saeed, MBBS1, Hamza Shahram, MBBS1, Nouman Shafique, MD13
1Rawalpindi Medical University, Rawalpindi, Punjab, Pakistan; 2Saidu Medical College Swat, Swat, North-West Frontier, Pakistan; 3King Edward Medical University, Lahore, Punjab, Pakistan; 4Liaquat University of Medical and Health Sciences, Jamshoro, Hyderabad, Sindh, Pakistan; 5Services Institute of Medical Sciences, Lahore, Punjab, Pakistan; 6Khyber Medical College, Peshawar, Mardan, North-West Frontier, Pakistan; 7Liaquat University of Medical and Health Science, Shahdadpur, Punjab, Pakistan; 8Pak Emirates Military Hospital, Rawalpindi, Taxila, Punjab, Pakistan; 9Fazaia Ruth Pfau Medical College, Bahawalpur, Punjab, Pakistan; 10Nishtar Medical University, Multan, Punjab, Pakistan; 11Saidu Medical College Swat, Mingora (SWAT), North-West Frontier, Pakistan; 12Foundation University Medical College, Islamabad, Islamabad, Pakistan; 13AdventHealth Medical Group, AdventHealth, Orlando, FL
Introduction: Ustekinumab (UST), an IL-12/23 antagonist, has been approved for treating patients with inflammatory bowel disease (IBD). For patients with inadequate response or loss of response to UST, dose escalation may be a promising option. Our meta-analysis evaluates the efficacy of dose escalation (via interval shortening or intravenous reinduction) of UST in patients with IBD.
Methods: A systematic literature search was done on MEDLINE, Embase, Cochrane, and Clinicaltrials.gov from inception to June 01, 2024, using MeSH terms and keywords for ‘Inflammatory Bowel Disease’ and ‘Ustekinumab’. Outcomes of interest were clinical response, clinical remission, steroid-free remission, endoscopic response, endoscopic remission, remission of perianal disease, progression to surgery, and normalization of CRP and fecal calprotectin following dose escalation. The random-effects model with the Freeman Tukey double arcsine transformation was used to pool overall prevalence with 95% CI for dichotomous variables in MetaXL version 5.3.
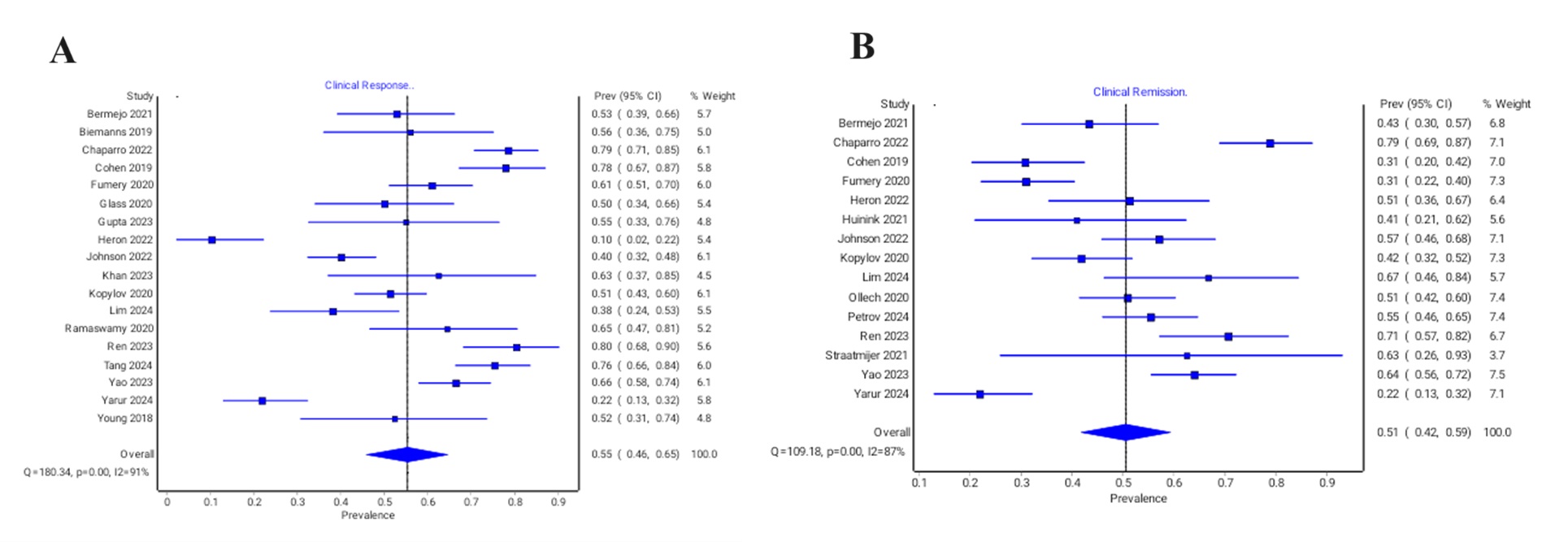
Results: We included data from, 28 studies, containing 2,030 patients. The median age of the patients ranged from 21.5-51.5 years. The pooled prevalence of patients reporting clinical response was 55% (95% CI: 0.46-0.65, I2=91%). Clinical remission and steroid free remission were reported in 51% (95% CI: 0.42-0.59, I2=87%) and 54% (95% CI: 0.45-0.64, I2=84%), respectively. Endoscopic response and endoscopic remission were achieved in 54% (95% CI: 0.42-0.65, I2=82%) and 33% (95% CI: 0.24-0.42, I2=73%), respectively. The pooled prevalence of patients reporting normalization of CRP, progression to surgery, remission of perianal disease and decrease in fecal calprotectin were 42% (95% CI: 0.33-0.52, I2=84%), 8% (95% CI: 0.05-0.12, I2=57%), 32% (95% CI: 0.22-0.43, I2=39%) and 54% (95% CI: 0.41-0.61, I2=75%), respectively.
Discussion: The pooled real world data suggest that dose escalation of ustekinumab is an efficacious option, with clinical and endoscopic response being achieved in 55% and 54% of patients, respectively. Further research is warranted to identify specific populations that are likely to benefit the most from dose escalation.
Disclosures:
Mohammad Ebad Ur Rehman, MBBS1, Abdul Qadeer Khan, MBBS2, Maha Sajjad, MBBS3, Ammara Tahir, MBBS4, Abu Huraira Bin Gulzar, 5, Aizaz Ali, MBBS6, Amna Hussain, MBBS7, Fatimah Shahid, MBBS1, Shahroon Zahid, MBBS8, Talha Bin. Yasin, MBBS9, Aqsa Bilal, MBBS10, Umara Rajput, MBBS11, Tehreem Fatima, MBBS12, Tehseen Haider, MBBS1, Sajeel Saeed, MBBS1, Hamza Shahram, MBBS1, Nouman Shafique, MD13. P2563 - Efficacy of Dose Escalation of Ustekinumab in Inflammatory Bowel Disease: A Systematic Review and Meta-Analysis, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
1Rawalpindi Medical University, Rawalpindi, Punjab, Pakistan; 2Saidu Medical College Swat, Swat, North-West Frontier, Pakistan; 3King Edward Medical University, Lahore, Punjab, Pakistan; 4Liaquat University of Medical and Health Sciences, Jamshoro, Hyderabad, Sindh, Pakistan; 5Services Institute of Medical Sciences, Lahore, Punjab, Pakistan; 6Khyber Medical College, Peshawar, Mardan, North-West Frontier, Pakistan; 7Liaquat University of Medical and Health Science, Shahdadpur, Punjab, Pakistan; 8Pak Emirates Military Hospital, Rawalpindi, Taxila, Punjab, Pakistan; 9Fazaia Ruth Pfau Medical College, Bahawalpur, Punjab, Pakistan; 10Nishtar Medical University, Multan, Punjab, Pakistan; 11Saidu Medical College Swat, Mingora (SWAT), North-West Frontier, Pakistan; 12Foundation University Medical College, Islamabad, Islamabad, Pakistan; 13AdventHealth Medical Group, AdventHealth, Orlando, FL
Introduction: Ustekinumab (UST), an IL-12/23 antagonist, has been approved for treating patients with inflammatory bowel disease (IBD). For patients with inadequate response or loss of response to UST, dose escalation may be a promising option. Our meta-analysis evaluates the efficacy of dose escalation (via interval shortening or intravenous reinduction) of UST in patients with IBD.
Methods: A systematic literature search was done on MEDLINE, Embase, Cochrane, and Clinicaltrials.gov from inception to June 01, 2024, using MeSH terms and keywords for ‘Inflammatory Bowel Disease’ and ‘Ustekinumab’. Outcomes of interest were clinical response, clinical remission, steroid-free remission, endoscopic response, endoscopic remission, remission of perianal disease, progression to surgery, and normalization of CRP and fecal calprotectin following dose escalation. The random-effects model with the Freeman Tukey double arcsine transformation was used to pool overall prevalence with 95% CI for dichotomous variables in MetaXL version 5.3.
Results: We included data from, 28 studies, containing 2,030 patients. The median age of the patients ranged from 21.5-51.5 years. The pooled prevalence of patients reporting clinical response was 55% (95% CI: 0.46-0.65, I2=91%). Clinical remission and steroid free remission were reported in 51% (95% CI: 0.42-0.59, I2=87%) and 54% (95% CI: 0.45-0.64, I2=84%), respectively. Endoscopic response and endoscopic remission were achieved in 54% (95% CI: 0.42-0.65, I2=82%) and 33% (95% CI: 0.24-0.42, I2=73%), respectively. The pooled prevalence of patients reporting normalization of CRP, progression to surgery, remission of perianal disease and decrease in fecal calprotectin were 42% (95% CI: 0.33-0.52, I2=84%), 8% (95% CI: 0.05-0.12, I2=57%), 32% (95% CI: 0.22-0.43, I2=39%) and 54% (95% CI: 0.41-0.61, I2=75%), respectively.
Discussion: The pooled real world data suggest that dose escalation of ustekinumab is an efficacious option, with clinical and endoscopic response being achieved in 55% and 54% of patients, respectively. Further research is warranted to identify specific populations that are likely to benefit the most from dose escalation.

Figure: Forest plots of (A) Clinical response and (B) Clinical remission
Disclosures:
Mohammad Ebad Ur Rehman indicated no relevant financial relationships.
Abdul Qadeer Khan indicated no relevant financial relationships.
Maha Sajjad indicated no relevant financial relationships.
Ammara Tahir indicated no relevant financial relationships.
Abu Huraira Bin Gulzar indicated no relevant financial relationships.
Aizaz Ali indicated no relevant financial relationships.
Amna Hussain indicated no relevant financial relationships.
Fatimah Shahid indicated no relevant financial relationships.
Shahroon Zahid indicated no relevant financial relationships.
Talha Yasin indicated no relevant financial relationships.
Aqsa Bilal indicated no relevant financial relationships.
Umara Rajput indicated no relevant financial relationships.
Tehreem Fatima indicated no relevant financial relationships.
Tehseen Haider indicated no relevant financial relationships.
Sajeel Saeed indicated no relevant financial relationships.
Hamza Shahram indicated no relevant financial relationships.
Nouman Shafique indicated no relevant financial relationships.
Mohammad Ebad Ur Rehman, MBBS1, Abdul Qadeer Khan, MBBS2, Maha Sajjad, MBBS3, Ammara Tahir, MBBS4, Abu Huraira Bin Gulzar, 5, Aizaz Ali, MBBS6, Amna Hussain, MBBS7, Fatimah Shahid, MBBS1, Shahroon Zahid, MBBS8, Talha Bin. Yasin, MBBS9, Aqsa Bilal, MBBS10, Umara Rajput, MBBS11, Tehreem Fatima, MBBS12, Tehseen Haider, MBBS1, Sajeel Saeed, MBBS1, Hamza Shahram, MBBS1, Nouman Shafique, MD13. P2563 - Efficacy of Dose Escalation of Ustekinumab in Inflammatory Bowel Disease: A Systematic Review and Meta-Analysis, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
