Monday Poster Session
Category: GI Bleeding
P2471 - Prognosis and Characteristics of In-Hospital Major Bleeding Patients Taking Factor-Xa Inhibitors in Japan: REAL-J-Bleeding Study
Monday, October 28, 2024
10:30 AM - 4:00 PM ET
Location: Exhibit Hall E
Has Audio

Fumitaka Wada, PhD
AstraZeneca K.K.
Osaka, Osaka, Japan
Presenting Author(s)
Fumitaka Wada, PhD1, Shun Kohsaka, MD, PhD2, Mitsuru Hoshino, PhD1, Yoshifumi Arita, MSc, MPH1, Hitomi Motobayashi, 1, Naru Morita, MD, PhD1, Masahiro Yasaka, MD, PhD3
1AstraZeneca K.K., Osaka, Osaka, Japan; 2Keio University School of Medicine, Shinjuku-Ku, Tokyo, Japan; 3Fukuoka Neurosurgical Hospital, Fukuoka, Fukuoka, Japan
Introduction: Although factor-Xa inhibitors (FXa-I) are increasingly used to prevent thromboembolic complications, there are limited data on the details on FXa-I related hemorrhagic events. In particular, few studies have examined outcomes, incident of subsequent thromboembolic complications, and reinstallation of oral anticoagulants (OACs) after the bleeding events. In this study, we investigated the characteristics and prognosis of patients who were hospitalized with major bleeding events on FXa-I, prior to the approval of antidotes such as andexanet alfa in Japan.
Methods: REAL-J-Bleeding was a retrospective observational descriptive study that aimed to generate real-world evidence from an administrative hospital claims database (Medical Data Vision). For the present study, we analyzed patients who were hospitalized with major bleeding while taking FXa-I between 2011-2022. The index date was defined as the hospitalization date of any first major bleeding event after prescription of FXa-I. The outcomes of interest were the proportion of patients who experienced thromboembolic events, hospital death during the hospitalization, and the resumption of OAC. The confidence interval (CI) was calculated by using the Clopper-Pearson exact method.
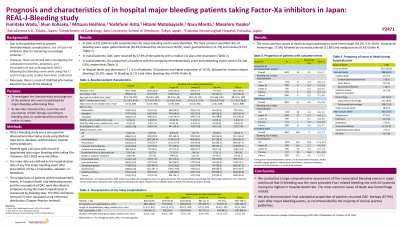
Results: In total, 8,011 patients (median age: 80.0 years, 62.4% males, rivaroxaban: 24.1%, apixaban: 34.2%, edoxaban: 41.7%) with hospitalization for major bleeding events were identified. The distribution of bleeding sites was as follows: upper gastrointestinal bleeding, 30.3%; intracranial hemorrhage, 16.0%; lower gastrointestinal bleeding, 11.7%; trauma-related bleeding, 9.1%; and other sites, 38.2%. During the hospitalization (median length of stay 11.0 days, range 1-911 days), the proportion of patients who experienced thromboembolic events and in-hospital death were 0.5% (95%CI: 0.3% to 0.7%) and 7.1% (95%CI: 6.6% to 7.7%), respectively. The most common cause of death was hemorrhage related. Among survivors, the proportion of OAC resumption was 67.9% and the median time to resumption was 3.0 days, range 1-216 days.
Discussion: FXa-I related major bleeding hospitalizations were associated with significant mortality. Furthermore, a substantial proportion of patients resumed OAC therapy even after major bleeding events, as recommended by the majority of clinical practice guidelines. These findings highlight the need for strategies to further improve outcomes in FXa-I related complications.
Disclosures:
Fumitaka Wada, PhD1, Shun Kohsaka, MD, PhD2, Mitsuru Hoshino, PhD1, Yoshifumi Arita, MSc, MPH1, Hitomi Motobayashi, 1, Naru Morita, MD, PhD1, Masahiro Yasaka, MD, PhD3. P2471 - Prognosis and Characteristics of In-Hospital Major Bleeding Patients Taking Factor-Xa Inhibitors in Japan: REAL-J-Bleeding Study, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
1AstraZeneca K.K., Osaka, Osaka, Japan; 2Keio University School of Medicine, Shinjuku-Ku, Tokyo, Japan; 3Fukuoka Neurosurgical Hospital, Fukuoka, Fukuoka, Japan
Introduction: Although factor-Xa inhibitors (FXa-I) are increasingly used to prevent thromboembolic complications, there are limited data on the details on FXa-I related hemorrhagic events. In particular, few studies have examined outcomes, incident of subsequent thromboembolic complications, and reinstallation of oral anticoagulants (OACs) after the bleeding events. In this study, we investigated the characteristics and prognosis of patients who were hospitalized with major bleeding events on FXa-I, prior to the approval of antidotes such as andexanet alfa in Japan.
Methods: REAL-J-Bleeding was a retrospective observational descriptive study that aimed to generate real-world evidence from an administrative hospital claims database (Medical Data Vision). For the present study, we analyzed patients who were hospitalized with major bleeding while taking FXa-I between 2011-2022. The index date was defined as the hospitalization date of any first major bleeding event after prescription of FXa-I. The outcomes of interest were the proportion of patients who experienced thromboembolic events, hospital death during the hospitalization, and the resumption of OAC. The confidence interval (CI) was calculated by using the Clopper-Pearson exact method.
Results: In total, 8,011 patients (median age: 80.0 years, 62.4% males, rivaroxaban: 24.1%, apixaban: 34.2%, edoxaban: 41.7%) with hospitalization for major bleeding events were identified. The distribution of bleeding sites was as follows: upper gastrointestinal bleeding, 30.3%; intracranial hemorrhage, 16.0%; lower gastrointestinal bleeding, 11.7%; trauma-related bleeding, 9.1%; and other sites, 38.2%. During the hospitalization (median length of stay 11.0 days, range 1-911 days), the proportion of patients who experienced thromboembolic events and in-hospital death were 0.5% (95%CI: 0.3% to 0.7%) and 7.1% (95%CI: 6.6% to 7.7%), respectively. The most common cause of death was hemorrhage related. Among survivors, the proportion of OAC resumption was 67.9% and the median time to resumption was 3.0 days, range 1-216 days.
Discussion: FXa-I related major bleeding hospitalizations were associated with significant mortality. Furthermore, a substantial proportion of patients resumed OAC therapy even after major bleeding events, as recommended by the majority of clinical practice guidelines. These findings highlight the need for strategies to further improve outcomes in FXa-I related complications.
Disclosures:
Fumitaka Wada: Astrazeneca – Employee.
Shun Kohsaka: Astrazeneca – Grant/Research Support. Bristol Myers Squibb – Grant/Research Support. Pfizer – Speakers Bureau.
Mitsuru Hoshino: Astrazeneca – Employee.
Yoshifumi Arita: Astrazeneca – Employee.
Hitomi Motobayashi: Astrazeneca – Employee.
Naru Morita: Astrazeneca – Employee.
Masahiro Yasaka: Astrazeneca – Speakers Bureau. Bayer – Speakers Bureau. Boehringer Ingelheim – Speakers Bureau. Bristol Myers Squibb – Speakers Bureau. Daiichi Sankyo – Speakers Bureau.
Fumitaka Wada, PhD1, Shun Kohsaka, MD, PhD2, Mitsuru Hoshino, PhD1, Yoshifumi Arita, MSc, MPH1, Hitomi Motobayashi, 1, Naru Morita, MD, PhD1, Masahiro Yasaka, MD, PhD3. P2471 - Prognosis and Characteristics of In-Hospital Major Bleeding Patients Taking Factor-Xa Inhibitors in Japan: REAL-J-Bleeding Study, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
