Monday Poster Session
Category: Colon
P1929 - Flavored Polyethylene Glycol and Sulfate Solution Is Safe in Cardiac and Hypertensive Patients Undergoing Colonoscopy: Results of a Post Hoc Analysis
Monday, October 28, 2024
10:30 AM - 4:00 PM ET
Location: Exhibit Hall E
Has Audio
- Mw
Matthew walker, PhD
Braintree Laboratories, Inc.
Braintree, MA
Presenting Author(s)
Douglas K. Rex, MD1, Jack DiPalma, MD2, Raj Bhandari, MD3, Matthew walker, PhD4, John McGowan, MPH4
1Indiana University School of Medicine, Carmel, IN; 2University of South Alabama, Mobile, AL; 3Delta Research Partners, Monroe, LA; 4Braintree Laboratories, Inc., Braintree, MA
Introduction: Flavored Polyethylene Glycol 3350 (PEG) and Sulfate Solution (FPSS) is an FDA approved colonic prep containing osmotic laxatives and supplemental salts to minimize diarrheal electrolyte losses. This post-hoc analysis of two Phase 3 studies evaluated the safety of FPSS in patients with and without a history of cardiac disorders and/or hypertension (HTN). In addition, comparisons to the FDA-approved control preps used in the studies (PEG plus Electrolytes and Ascorbate [PEG-EA] and Oral Sulfate Solution [OSS]) were also performed.
Methods: Adults undergoing colonoscopy for routine indications were included in this analysis. Patients with a clinically significant baseline ECG, a NYHA classification of 3/4, or uncontrolled HTN (systolic >170 mmHg, diastolic< 100 mmHg) were excluded from the study and not included in the analysis. All preps were administered following FDA approved split-dose (PM/AM) labeling. Vital signs and serum chemistry were evaluated at screening and post prep (prior to colonoscopy). Treatment-emergent adverse events (AEs) were collected via spontaneous patient reports.
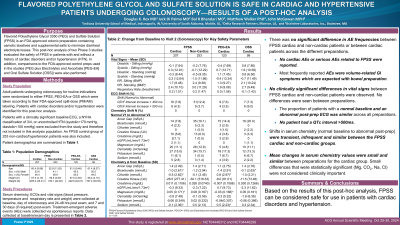
Results: 425 patients (mean age 62 years, 51% male) with a history of cardiac disorders and/or HTN (the “cardiac” group - FPSS 207, PEG-EA 120, OSS 99 patients) and 253 noncardiac patients (mean age 53, 41% male) given FPSS were evaluated. ECG parameters and vital signs were similar across all analysis groups. The proportion of patients with a normal baseline and an abnormal post-dosing ECG was similar between cardiac and non-cardiac FPSS patients and the control preps. No patient had a QT interval >500ms.
Transient shifts in serum chemistry (normal baseline to abnormal post-prep) were infrequent and similar between the FPSS cardiac and non-cardiac groups. Mean changes in serum chemistry values were small and consistent between preps for the cardiac group. The small differences that were statistically significant (Mg, CO2, Na, Cl) were not considered clinically important.
There was no significant difference in AE frequencies between FPSS cardiac and non-cardiac patients or between cardiac patients across the different prep groups. The most frequently reported AEs were volume-related GI symptoms which are expected with bowel preparation. No cardiac AEs or serious AEs related to FPSS were reported.
Discussion: Based on the results of this post-hoc analysis, FPSS can be considered safe for use in patients with cardiac disorders and hypertension.
Note: The table for this abstract can be viewed in the ePoster Gallery section of the ACG 2024 ePoster Site or in The American Journal of Gastroenterology's abstract supplement issue, both of which will be available starting October 27, 2024.
Disclosures:
Douglas K. Rex, MD1, Jack DiPalma, MD2, Raj Bhandari, MD3, Matthew walker, PhD4, John McGowan, MPH4. P1929 - Flavored Polyethylene Glycol and Sulfate Solution Is Safe in Cardiac and Hypertensive Patients Undergoing Colonoscopy: Results of a <i>Post Hoc</i> Analysis, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
1Indiana University School of Medicine, Carmel, IN; 2University of South Alabama, Mobile, AL; 3Delta Research Partners, Monroe, LA; 4Braintree Laboratories, Inc., Braintree, MA
Introduction: Flavored Polyethylene Glycol 3350 (PEG) and Sulfate Solution (FPSS) is an FDA approved colonic prep containing osmotic laxatives and supplemental salts to minimize diarrheal electrolyte losses. This post-hoc analysis of two Phase 3 studies evaluated the safety of FPSS in patients with and without a history of cardiac disorders and/or hypertension (HTN). In addition, comparisons to the FDA-approved control preps used in the studies (PEG plus Electrolytes and Ascorbate [PEG-EA] and Oral Sulfate Solution [OSS]) were also performed.
Methods: Adults undergoing colonoscopy for routine indications were included in this analysis. Patients with a clinically significant baseline ECG, a NYHA classification of 3/4, or uncontrolled HTN (systolic >170 mmHg, diastolic< 100 mmHg) were excluded from the study and not included in the analysis. All preps were administered following FDA approved split-dose (PM/AM) labeling. Vital signs and serum chemistry were evaluated at screening and post prep (prior to colonoscopy). Treatment-emergent adverse events (AEs) were collected via spontaneous patient reports.
Results: 425 patients (mean age 62 years, 51% male) with a history of cardiac disorders and/or HTN (the “cardiac” group - FPSS 207, PEG-EA 120, OSS 99 patients) and 253 noncardiac patients (mean age 53, 41% male) given FPSS were evaluated. ECG parameters and vital signs were similar across all analysis groups. The proportion of patients with a normal baseline and an abnormal post-dosing ECG was similar between cardiac and non-cardiac FPSS patients and the control preps. No patient had a QT interval >500ms.
Transient shifts in serum chemistry (normal baseline to abnormal post-prep) were infrequent and similar between the FPSS cardiac and non-cardiac groups. Mean changes in serum chemistry values were small and consistent between preps for the cardiac group. The small differences that were statistically significant (Mg, CO2, Na, Cl) were not considered clinically important.
There was no significant difference in AE frequencies between FPSS cardiac and non-cardiac patients or between cardiac patients across the different prep groups. The most frequently reported AEs were volume-related GI symptoms which are expected with bowel preparation. No cardiac AEs or serious AEs related to FPSS were reported.
Discussion: Based on the results of this post-hoc analysis, FPSS can be considered safe for use in patients with cardiac disorders and hypertension.
Note: The table for this abstract can be viewed in the ePoster Gallery section of the ACG 2024 ePoster Site or in The American Journal of Gastroenterology's abstract supplement issue, both of which will be available starting October 27, 2024.
Disclosures:
Douglas Rex: Acacia – Consultant. Boston Scientific – Consultant. Braintree Laboratories Inc – Consultant, Grant/Research Support. Erbe – Grant/Research Support. Medivators – Grant/Research Support. Medtronic – Consultant. Norgine – Consultant. Olympus Corporation – Consultant, Grant/Research Support. Satisfai Health – Stock Options.
Jack DiPalma: Sebela Pharmaceuticals – Consultant, Independent Contractor.
Raj Bhandari indicated no relevant financial relationships.
Matthew walker: Braintree, A Part of Sebela – Employee.
John McGowan: Braintree, A Part of Sebela – Employee.
Douglas K. Rex, MD1, Jack DiPalma, MD2, Raj Bhandari, MD3, Matthew walker, PhD4, John McGowan, MPH4. P1929 - Flavored Polyethylene Glycol and Sulfate Solution Is Safe in Cardiac and Hypertensive Patients Undergoing Colonoscopy: Results of a <i>Post Hoc</i> Analysis, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
