Sunday Poster Session
Category: Colon
P0370 - A Case of Endoscopic Full-Thickness Resection for a Known Colon Adenocarcinoma in a Patient With Decompensated Cirrhosis
Sunday, October 27, 2024
3:30 PM - 7:00 PM ET
Location: Exhibit Hall E
Has Audio
- SV
Shyam C. Vedantam, DO
University of Miami Miller School of Medicine
Miami, FL
Presenting Author(s)
Award: ACG Case Reports Journal Award (Trainee)
Award: Presidential Poster Award
Shyam C. Vedantam, DO1, Nicole Ferrante, MD2, Shria Kumar, MD, MSCE1, Sean Bhalla, MD1
1University of Miami Miller School of Medicine, Miami, FL; 2University of Miami, Miami, FL
Introduction: Endoscopic full-thickness resection (EFTR) is a novel approach for endoscopic resection of lesions when conventional techniques may not be feasible based on concern for perforation, incomplete resection, technically difficult location, or need for deeper resection. The full thickness resection device (FTRD) utilizes a preloaded system with Over-the-scope clips (OTSC) and snare to complete resections. We present a case of using the FTRD system for a cecal adenocarcinoma in a non-surgical candidate.
Case Description/Methods: A 77 year old male with a history of nonalcoholic steatohepatitis cirrhosis (complicated by esophageal varices and ablated hepatocellular carcinoma) underwent surveillance colonoscopy showing a 2.5cm Paris IIa cecal polyp. Hot snare polypectomy was incomplete due to difficulty lifting, and only a portion was resected. Pathology of the resected specimen showed adenocarcinoma. CT scan was negative for metastasis. Due to his decompensated cirrhosis (platelet count of 39,000, MELD score 20), he was deemed a high-risk surgical candidate by surgery and hepatology. A multidisciplinary decision was made to pursue EFTR. On repeat colonoscopy, the residual 18mm by 18mm cecal lesion was identified and demarcated. A colonoscope with the FTRD device was introduced and EFTR was performed. Visible omental fat suggested full thickness resection. A small irregular area of polypoid tissue that was overlying the Ovesco clip was resected by snare. There were no complications. Pathology showed adenocarcinoma invading the submucosa and extending to the lateral tissue edge (resected with hot snare) and lymphovascular invasion, though the tumor was completely resected. Given comorbidities, palliative chemotherapy was offered, but the patient opted for surveillance. He is due for follow-up to determine the presence of residual carcinoma.
Discussion: Prior studies have shown FTRD can have technical and clinical success. Its utility in patients with cirrhosis has not been fully studied. This is a novel case with mitigating factors that favored use of FTRD. The depth and invasive nature of this malignant lesion prevented endoscopic mucosal resection or dissection. This case highlights that this device can safely perform resections as an alternative for patients who are at high risk for surgery and provide diagnostic information that can guide further oncologic care.
Disclosures:
Shyam C. Vedantam, DO1, Nicole Ferrante, MD2, Shria Kumar, MD, MSCE1, Sean Bhalla, MD1. P0370 - A Case of Endoscopic Full-Thickness Resection for a Known Colon Adenocarcinoma in a Patient With Decompensated Cirrhosis, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
Award: Presidential Poster Award
Shyam C. Vedantam, DO1, Nicole Ferrante, MD2, Shria Kumar, MD, MSCE1, Sean Bhalla, MD1
1University of Miami Miller School of Medicine, Miami, FL; 2University of Miami, Miami, FL
Introduction: Endoscopic full-thickness resection (EFTR) is a novel approach for endoscopic resection of lesions when conventional techniques may not be feasible based on concern for perforation, incomplete resection, technically difficult location, or need for deeper resection. The full thickness resection device (FTRD) utilizes a preloaded system with Over-the-scope clips (OTSC) and snare to complete resections. We present a case of using the FTRD system for a cecal adenocarcinoma in a non-surgical candidate.
Case Description/Methods: A 77 year old male with a history of nonalcoholic steatohepatitis cirrhosis (complicated by esophageal varices and ablated hepatocellular carcinoma) underwent surveillance colonoscopy showing a 2.5cm Paris IIa cecal polyp. Hot snare polypectomy was incomplete due to difficulty lifting, and only a portion was resected. Pathology of the resected specimen showed adenocarcinoma. CT scan was negative for metastasis. Due to his decompensated cirrhosis (platelet count of 39,000, MELD score 20), he was deemed a high-risk surgical candidate by surgery and hepatology. A multidisciplinary decision was made to pursue EFTR. On repeat colonoscopy, the residual 18mm by 18mm cecal lesion was identified and demarcated. A colonoscope with the FTRD device was introduced and EFTR was performed. Visible omental fat suggested full thickness resection. A small irregular area of polypoid tissue that was overlying the Ovesco clip was resected by snare. There were no complications. Pathology showed adenocarcinoma invading the submucosa and extending to the lateral tissue edge (resected with hot snare) and lymphovascular invasion, though the tumor was completely resected. Given comorbidities, palliative chemotherapy was offered, but the patient opted for surveillance. He is due for follow-up to determine the presence of residual carcinoma.
Discussion: Prior studies have shown FTRD can have technical and clinical success. Its utility in patients with cirrhosis has not been fully studied. This is a novel case with mitigating factors that favored use of FTRD. The depth and invasive nature of this malignant lesion prevented endoscopic mucosal resection or dissection. This case highlights that this device can safely perform resections as an alternative for patients who are at high risk for surgery and provide diagnostic information that can guide further oncologic care.

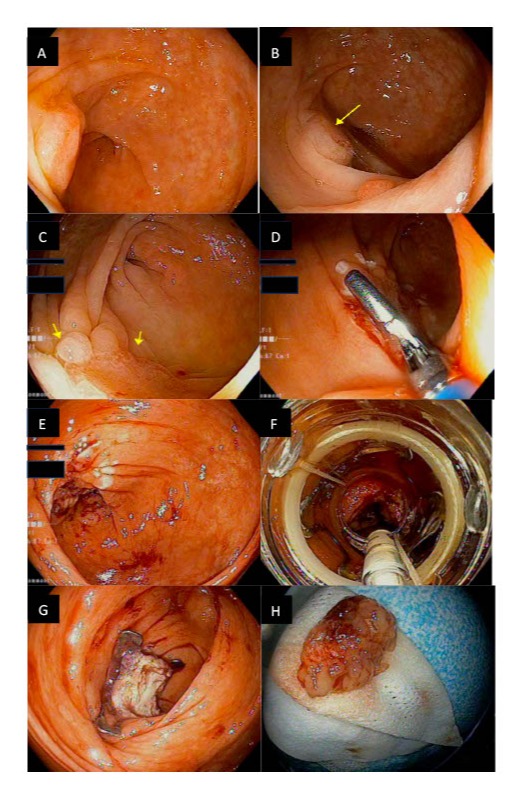
Figure: A: Image from first colonoscopy identifying 25 mm Paris IIa cecal polyp.
B: Image from first colonoscopy identifying 25 mm Paris IIa cecal polyp (identified by yellow arrow).
C: Image from the second colonoscopy reidentifying the 18 mm mass in the cecum that was scarred from prior resection.
D: Image from the second colonoscopy showing the thermal demarcation process to define the borders of the lesion.
E: Image from the second colonoscopy showing the lesion after the demarcation process.
F: Image from the second colonoscopy showing the view with the full thickness resection device fitted onto the colonoscope with the lesion in the center of the screen.
G: Image from the second colonoscopy showing the post-polypectomy site, Ovesco clip in place, and visible omental fat.
H: Complete resection and retrieval of the polyp tissue.
B: Image from first colonoscopy identifying 25 mm Paris IIa cecal polyp (identified by yellow arrow).
C: Image from the second colonoscopy reidentifying the 18 mm mass in the cecum that was scarred from prior resection.
D: Image from the second colonoscopy showing the thermal demarcation process to define the borders of the lesion.
E: Image from the second colonoscopy showing the lesion after the demarcation process.
F: Image from the second colonoscopy showing the view with the full thickness resection device fitted onto the colonoscope with the lesion in the center of the screen.
G: Image from the second colonoscopy showing the post-polypectomy site, Ovesco clip in place, and visible omental fat.
H: Complete resection and retrieval of the polyp tissue.
Disclosures:
Shyam Vedantam indicated no relevant financial relationships.
Nicole Ferrante indicated no relevant financial relationships.
Shria Kumar indicated no relevant financial relationships.
Sean Bhalla indicated no relevant financial relationships.
Shyam C. Vedantam, DO1, Nicole Ferrante, MD2, Shria Kumar, MD, MSCE1, Sean Bhalla, MD1. P0370 - A Case of Endoscopic Full-Thickness Resection for a Known Colon Adenocarcinoma in a Patient With Decompensated Cirrhosis, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.


