Sunday Poster Session
Category: Esophagus
P0479 - Dupilumab Is Effective in Treating EoE in Patients Weighing ≥15 kg: Results From the Phase 3 KIDS Study
Sunday, October 27, 2024
3:30 PM - 7:00 PM ET
Location: Exhibit Hall E
Has Audio
- MC
Mirna Chehade, MD, MPH
Icahn School of Medicine at Mount Sinai
New York, NY
Presenting Author(s)
Award: Presidential Poster Award
Mirna Chehade, MD, MPH1, Antonella Cianferoni, MD, PhD2, Changming Xia, PhD3, Tiffany Pela, PharmD, MPH4, Bram P. Raphael, MD3, Amr Radwan, MA, MBBCh3, Juby A. Jacob-Nara, MBBS, MD, MPH, MBA4
1Icahn School of Medicine at Mount Sinai, New York, NY; 2Perelman School of Medicine at the University of Pennsylvania, Philadelphia, PA; 3Regeneron Pharmaceuticals Inc., Tarrytown, NY; 4Sanofi, Bridgewater, NJ
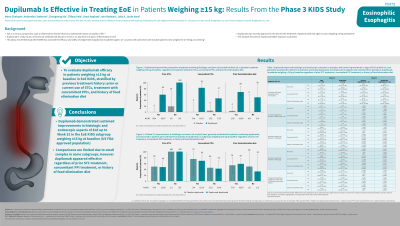
Introduction: Eosinophilic esophagitis (EoE) is a chronic, progressive, type 2 inflammatory disease which has a substantial impact on quality of life. Although EoE has similar histology in children and adults, symptoms in children tend to be more heterogeneous and non-specific than in adults. Dupilumab (DPL) is a fully human monoclonal antibody that blocks interleukin (IL)-4 and IL-13, key drivers of type 2 inflammation in EoE. The phase 3 EoE KIDS trial (NCT04394351) assessed efficacy of DPL in patients (pts) aged 1 to < 12 years with active EoE, and included pts weighing 5 to 60 kg. DPL was recently approved in the USA for the treatment of pts with EoE aged ≥1 year, weighing ≥15 kg. The objective of this analysis was to evaluate DPL efficacy by previous treatment history, focusing on the approved pediatric population weighing ≥15 kg.
Methods: The study comprised a double-blind placebo (PBO)-controlled period (Part A) where pts were randomized 2:2:1:1 to receive DPL higher-exposure (HE) or lower-exposure (LE), or PBO (2 groups) for 16 weeks (wks), and a 36-wk extension period where all pts received HE or LE DPL (Part B). Endpoints evaluated in this analysis were the primary endpoint of the KIDS study; proportion of pts achieving ≤6 eosinophils per high-powered field (eos/hpf); and two key secondary endpoints; < 15 eos/hpf, and Endoscopic Reference Score (EREFS) scores. Treatment efficacy was analysed in 3 subgroups: prior use of swallowed topical corticosteroids (STCs), treatment with concomitant proton-pump inhibitors (PPIs), and history of food elimination (FE) diet.
Results: In pediatric pts weighing ≥15 kg, DPL HE improved rates of histologic remission vs PBO at Wk 16, regardless of prior STC use (yes: 59.3% vs 0.0%, no: 100.0% vs 20.0%), treatment with concomitant PPIs (yes: 82.4% vs 0.0%, no: 46.7% vs 4.8%), or history of FE diet (yes: 67.9% vs 4.0%, no: 50.0% vs 0.0%; Table). Responses were maintained at Wk 52 in pts continuing DPL HE and improved in pts who switched from PBO to DPL HE, regardless of prior STC use, treatment with concomitant PPIs, or history of FE diet, although pt numbers in some subgroups were small (Table). A similar pattern of improvement regardless of subgroup was observed in pts achieving < 15 eos/hpf and in total EREFS score, in pts weighing ≥15 kg (Table). DPL was generally well tolerated.
Discussion: DPL demonstrated improvements vs PBO in histologic and endoscopic aspects of EoE in the ≥15 kg population up to Wk 52, regardless of prior treatment history.
Note: The table for this abstract can be viewed in the ePoster Gallery section of the ACG 2024 ePoster Site or in The American Journal of Gastroenterology's abstract supplement issue, both of which will be available starting October 27, 2024.
Disclosures:
Mirna Chehade, MD, MPH1, Antonella Cianferoni, MD, PhD2, Changming Xia, PhD3, Tiffany Pela, PharmD, MPH4, Bram P. Raphael, MD3, Amr Radwan, MA, MBBCh3, Juby A. Jacob-Nara, MBBS, MD, MPH, MBA4. P0479 - Dupilumab Is Effective in Treating EoE in Patients Weighing ≥15 kg: Results From the Phase 3 KIDS Study, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
Mirna Chehade, MD, MPH1, Antonella Cianferoni, MD, PhD2, Changming Xia, PhD3, Tiffany Pela, PharmD, MPH4, Bram P. Raphael, MD3, Amr Radwan, MA, MBBCh3, Juby A. Jacob-Nara, MBBS, MD, MPH, MBA4
1Icahn School of Medicine at Mount Sinai, New York, NY; 2Perelman School of Medicine at the University of Pennsylvania, Philadelphia, PA; 3Regeneron Pharmaceuticals Inc., Tarrytown, NY; 4Sanofi, Bridgewater, NJ
Introduction: Eosinophilic esophagitis (EoE) is a chronic, progressive, type 2 inflammatory disease which has a substantial impact on quality of life. Although EoE has similar histology in children and adults, symptoms in children tend to be more heterogeneous and non-specific than in adults. Dupilumab (DPL) is a fully human monoclonal antibody that blocks interleukin (IL)-4 and IL-13, key drivers of type 2 inflammation in EoE. The phase 3 EoE KIDS trial (NCT04394351) assessed efficacy of DPL in patients (pts) aged 1 to < 12 years with active EoE, and included pts weighing 5 to 60 kg. DPL was recently approved in the USA for the treatment of pts with EoE aged ≥1 year, weighing ≥15 kg. The objective of this analysis was to evaluate DPL efficacy by previous treatment history, focusing on the approved pediatric population weighing ≥15 kg.
Methods: The study comprised a double-blind placebo (PBO)-controlled period (Part A) where pts were randomized 2:2:1:1 to receive DPL higher-exposure (HE) or lower-exposure (LE), or PBO (2 groups) for 16 weeks (wks), and a 36-wk extension period where all pts received HE or LE DPL (Part B). Endpoints evaluated in this analysis were the primary endpoint of the KIDS study; proportion of pts achieving ≤6 eosinophils per high-powered field (eos/hpf); and two key secondary endpoints; < 15 eos/hpf, and Endoscopic Reference Score (EREFS) scores. Treatment efficacy was analysed in 3 subgroups: prior use of swallowed topical corticosteroids (STCs), treatment with concomitant proton-pump inhibitors (PPIs), and history of food elimination (FE) diet.
Results: In pediatric pts weighing ≥15 kg, DPL HE improved rates of histologic remission vs PBO at Wk 16, regardless of prior STC use (yes: 59.3% vs 0.0%, no: 100.0% vs 20.0%), treatment with concomitant PPIs (yes: 82.4% vs 0.0%, no: 46.7% vs 4.8%), or history of FE diet (yes: 67.9% vs 4.0%, no: 50.0% vs 0.0%; Table). Responses were maintained at Wk 52 in pts continuing DPL HE and improved in pts who switched from PBO to DPL HE, regardless of prior STC use, treatment with concomitant PPIs, or history of FE diet, although pt numbers in some subgroups were small (Table). A similar pattern of improvement regardless of subgroup was observed in pts achieving < 15 eos/hpf and in total EREFS score, in pts weighing ≥15 kg (Table). DPL was generally well tolerated.
Discussion: DPL demonstrated improvements vs PBO in histologic and endoscopic aspects of EoE in the ≥15 kg population up to Wk 52, regardless of prior treatment history.
Note: The table for this abstract can be viewed in the ePoster Gallery section of the ACG 2024 ePoster Site or in The American Journal of Gastroenterology's abstract supplement issue, both of which will be available starting October 27, 2024.
Disclosures:
Mirna Chehade: Adare/Ellodi – Consultant, Grant/Research Support. Allakos – Consultant, Grant/Research Support. AstraZeneca – Consultant, Grant/Research Support. Bristol Myers Squibb – Consultant, Grant/Research Support. Danone – Grant/Research Support. Nexstone Immunology – Consultant. Phathom – Consultant. Recludix Pharma – Consultant. Regeneron Pharmaceuticals Inc. – Consultant, Grant/Research Support. Sanofi – Consultant. Shire/Takeda – Consultant, Grant/Research Support.
Antonella Cianferoni: Aimmune – Grant/Research Support. AstraZeneca – Consultant. DBV Technologies – Advisory Committee/Board Member, Grant/Research Support. Regeneron Pharmaceuticals Inc. – Advisory Committee/Board Member. Sanofi – Advisory Committee/Board Member.
Changming Xia: Regeneron Pharmaceuticals Inc. – Employee, Stock-publicly held company(excluding mutual/index funds).
Tiffany Pela: Sanofi – Employee, Stock Options.
Bram P. Raphael: Regeneron Pharmaceuticals Inc. – Employee, Stock-publicly held company(excluding mutual/index funds).
Amr Radwan: Regeneron Pharmaceuticals Inc. – Employee, Stock-publicly held company(excluding mutual/index funds).
Juby A. Jacob-Nara: Sanofi – Employee, Stock Options.
Mirna Chehade, MD, MPH1, Antonella Cianferoni, MD, PhD2, Changming Xia, PhD3, Tiffany Pela, PharmD, MPH4, Bram P. Raphael, MD3, Amr Radwan, MA, MBBCh3, Juby A. Jacob-Nara, MBBS, MD, MPH, MBA4. P0479 - Dupilumab Is Effective in Treating EoE in Patients Weighing ≥15 kg: Results From the Phase 3 KIDS Study, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.

