Sunday Poster Session
Category: Esophagus
P0480 - Dupilumab Efficacy in Children With Eosinophilic Esophagitis and Prior Use of or Prior Inadequate Response, Intolerance, or Contraindication to Swallowed Topical Corticosteroids: Results From the Phase 3 EoE KIDS Study
Sunday, October 27, 2024
3:30 PM - 7:00 PM ET
Location: Exhibit Hall E
Has Audio
- MC
Mirna Chehade, MD, MPH
Icahn School of Medicine at Mount Sinai
New York, NY
Presenting Author(s)
Award: Presidential Poster Award
Mirna Chehade, MD, MPH1, Salvatore Oliva, MD, PhD2, Dhandapani Ashok, MBBS, MD3, Ruiqi Liu, PhD4, Jennifer Maloney, MD4, Raolat M. Abdulai, MD, MSc5, Margee Louisias, MD, MPH5, Allen Radin, MD4
1Icahn School of Medicine at Mount Sinai, New York, NY; 2University Hospital Umberto, Sapienza University of Rome, Rome, Lazio, Italy; 3Western University, London, ON, Canada; 4Regeneron Pharmaceuticals Inc., Tarrytown, NY; 5Sanofi, Bridgewater, NJ
Introduction: Eosinophilic esophagitis (EoE) is a chronic type 2 inflammatory disease of the esophagus. Swallowed topical corticosteroids (STCs) are commonly used to treat EoE, but not all patients achieve remission and others may be intolerant or have contraindications. Dupilumab is approved for EoE in patients aged ≥1 year, weighing ≥15 kg, in the US. We assessed the effect of dupilumab in children aged 1 to < 12 years with EoE with prior use of or prior inadequate response (IR)/intolerance/contraindication to STCs in the Phase 3 EoE KIDS study (NCT04394351).
Methods: This analysis includes patients randomized at Part A baseline to weight-tiered dupilumab higher-exposure (HE) or pooled placebo (PBO) up to Week (W) 16. Part B data were analyzed for patients who continued dupilumab HE, or switched from PBO to dupilumab HE through to W52. STC use ≤8 weeks before study baseline was prohibited. The primary endpoint was the proportion of patients achieving histologic remission (peak eosinophil count [PEC] ≤6 eosinophils/high-power field [eos/hpf]) at W16. Secondary endpoints were the proportion of patients achieving PEC < 15 eos/hpf, percent change in PEC, absolute change in Endoscopic Reference Score, EoE-Histologic Scoring System grade/stage scores, and proportion of days with ≥1 EoE sign as assessed by the Pediatric EoE Sign/Symptom Questionnaire – Caregiver version. Efficacy was assessed by prior use of or prior IR/intolerance/contraindication to STCs.
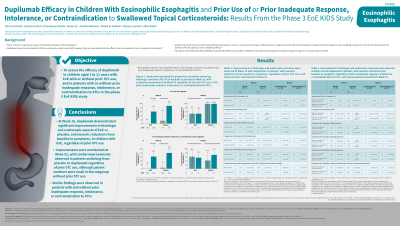
Results: Of the 102 patients who entered the study, most (80.4%) had a history of STC therapy, and 57.8% had a prior IR/intolerance/contraindication to STCs. Regardless of prior STC use, dupilumab HE improved rates of histologic remission and key secondary endpoints vs PBO at W16 (Table). Responses were maintained at W52 in patients continuing dupilumab HE, and improved in patients who switched from PBO to dupilumab HE regardless of prior STC use, although patient numbers were small in the subgroup without prior STC use (Table). Similar improvements in primary and secondary endpoints were observed in patients with and without prior IR/intolerance/contraindication to STCs.
Discussion: Dupilumab HE showed sustained improvements in histologic and endoscopic aspects of EoE vs PBO, and numerical improvements in symptoms, in children with EoE regardless of prior use of or prior IR/intolerance/contraindication to STCs.
Note: The table for this abstract can be viewed in the ePoster Gallery section of the ACG 2024 ePoster Site or in The American Journal of Gastroenterology's abstract supplement issue, both of which will be available starting October 27, 2024.
Disclosures:
Mirna Chehade, MD, MPH1, Salvatore Oliva, MD, PhD2, Dhandapani Ashok, MBBS, MD3, Ruiqi Liu, PhD4, Jennifer Maloney, MD4, Raolat M. Abdulai, MD, MSc5, Margee Louisias, MD, MPH5, Allen Radin, MD4. P0480 - Dupilumab Efficacy in Children With Eosinophilic Esophagitis and Prior Use of or Prior Inadequate Response, Intolerance, or Contraindication to Swallowed Topical Corticosteroids: Results From the Phase 3 EoE KIDS Study, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
Mirna Chehade, MD, MPH1, Salvatore Oliva, MD, PhD2, Dhandapani Ashok, MBBS, MD3, Ruiqi Liu, PhD4, Jennifer Maloney, MD4, Raolat M. Abdulai, MD, MSc5, Margee Louisias, MD, MPH5, Allen Radin, MD4
1Icahn School of Medicine at Mount Sinai, New York, NY; 2University Hospital Umberto, Sapienza University of Rome, Rome, Lazio, Italy; 3Western University, London, ON, Canada; 4Regeneron Pharmaceuticals Inc., Tarrytown, NY; 5Sanofi, Bridgewater, NJ
Introduction: Eosinophilic esophagitis (EoE) is a chronic type 2 inflammatory disease of the esophagus. Swallowed topical corticosteroids (STCs) are commonly used to treat EoE, but not all patients achieve remission and others may be intolerant or have contraindications. Dupilumab is approved for EoE in patients aged ≥1 year, weighing ≥15 kg, in the US. We assessed the effect of dupilumab in children aged 1 to < 12 years with EoE with prior use of or prior inadequate response (IR)/intolerance/contraindication to STCs in the Phase 3 EoE KIDS study (NCT04394351).
Methods: This analysis includes patients randomized at Part A baseline to weight-tiered dupilumab higher-exposure (HE) or pooled placebo (PBO) up to Week (W) 16. Part B data were analyzed for patients who continued dupilumab HE, or switched from PBO to dupilumab HE through to W52. STC use ≤8 weeks before study baseline was prohibited. The primary endpoint was the proportion of patients achieving histologic remission (peak eosinophil count [PEC] ≤6 eosinophils/high-power field [eos/hpf]) at W16. Secondary endpoints were the proportion of patients achieving PEC < 15 eos/hpf, percent change in PEC, absolute change in Endoscopic Reference Score, EoE-Histologic Scoring System grade/stage scores, and proportion of days with ≥1 EoE sign as assessed by the Pediatric EoE Sign/Symptom Questionnaire – Caregiver version. Efficacy was assessed by prior use of or prior IR/intolerance/contraindication to STCs.
Results: Of the 102 patients who entered the study, most (80.4%) had a history of STC therapy, and 57.8% had a prior IR/intolerance/contraindication to STCs. Regardless of prior STC use, dupilumab HE improved rates of histologic remission and key secondary endpoints vs PBO at W16 (Table). Responses were maintained at W52 in patients continuing dupilumab HE, and improved in patients who switched from PBO to dupilumab HE regardless of prior STC use, although patient numbers were small in the subgroup without prior STC use (Table). Similar improvements in primary and secondary endpoints were observed in patients with and without prior IR/intolerance/contraindication to STCs.
Discussion: Dupilumab HE showed sustained improvements in histologic and endoscopic aspects of EoE vs PBO, and numerical improvements in symptoms, in children with EoE regardless of prior use of or prior IR/intolerance/contraindication to STCs.
Note: The table for this abstract can be viewed in the ePoster Gallery section of the ACG 2024 ePoster Site or in The American Journal of Gastroenterology's abstract supplement issue, both of which will be available starting October 27, 2024.
Disclosures:
Mirna Chehade: Adare/Ellodi – Consultant, Grant/Research Support. Allakos – Consultant, Grant/Research Support. AstraZeneca – Consultant, Grant/Research Support. Bristol Myers Squibb – Consultant, Grant/Research Support. Danone – Grant/Research Support. Nexstone Immunology – Consultant. Phathom – Consultant. Recludix Pharma – Consultant. Regeneron Pharmaceuticals Inc. – Consultant, Grant/Research Support. Sanofi – Consultant. Shire/Takeda – Consultant, Grant/Research Support.
Salvatore Oliva: Celgene/Receptos/BMS – Advisory Committee/Board Member, Consultant. Medtronic – Advisory Committee/Board Member, Consultant, Speakers Bureau. Ocean Pharma – Advisory Committee/Board Member, Consultant. Sanofi/Regeneron Pharmaceuticals Inc. – Advisory Committee/Board Member, Consultant, Speakers Bureau.
Dhandapani Ashok: Alexion Pharma – Consultant. LaunchitDTx – Consultant. Mirum Pharma – Consultant. Sanofi – Speakers Bureau.
Ruiqi Liu: Regeneron Pharmaceuticals Inc. – Employee, Stock-publicly held company(excluding mutual/index funds).
Jennifer Maloney: Regeneron Pharmaceuticals Inc. – Employee, Stock-publicly held company(excluding mutual/index funds).
Raolat M. Abdulai: Sanofi – Employee, Stock Options.
Margee Louisias: Sanofi – Employee, Stock Options.
Allen Radin: Regeneron Pharmaceuticals Inc. – Employee, Stock-publicly held company(excluding mutual/index funds).
Mirna Chehade, MD, MPH1, Salvatore Oliva, MD, PhD2, Dhandapani Ashok, MBBS, MD3, Ruiqi Liu, PhD4, Jennifer Maloney, MD4, Raolat M. Abdulai, MD, MSc5, Margee Louisias, MD, MPH5, Allen Radin, MD4. P0480 - Dupilumab Efficacy in Children With Eosinophilic Esophagitis and Prior Use of or Prior Inadequate Response, Intolerance, or Contraindication to Swallowed Topical Corticosteroids: Results From the Phase 3 EoE KIDS Study, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.

