Sunday Poster Session
Category: Functional Bowel Disease
P0627 - Efficacy and Safety of Plecanatide in Treatment of Irritable Bowel Syndrome With Constipation and Chronic Idiopathic Constipation
Sunday, October 27, 2024
3:30 PM - 7:00 PM ET
Location: Exhibit Hall E
Has Audio

Iyad Al-Bustami, MD, MPH(c)
Brooklyn Hospital Center
Brooklyn, NY
Presenting Author(s)
Shahzaib Ahmed, MBBS1, Eeman Ahmad, MBBS1, Umar Akram, 2, Iyad Al-bustami, MD, MPH(c)3
1Fatima Memorial Hospital College of Medicine and Dentistry, Lahore, Punjab, Pakistan; 2Allama Iqbal Medical College, Lahore, Punjab, Pakistan; 3Brooklyn Hospital Center, Houston, TX
Introduction: Plecanatide is a guanylate cyclase-C agonist that is indicated for the treatment of irritable bowel syndrome with constipation (IBS-C) and chronic idiopathic constipation (CIC).
Methods: We searched through PubMed, Cochrane CENTRAL, Scopus, and ClinicalTrials.gov from inception until June 2024. Trials comparing plecanatide with placebo in patients with IBS-C or CIC were included. Random effects model was used and risk ratios (RRs) and mean differences (MDs) were calculated with their 95% confidence intervals (CIs) using R version 4.4.0. Publication bias was assessed by Luis Furuya-Kanamori index and doi plots.
Results: A total of 7 studies consisting of 6316 participants were included in the analysis. 4349 participants received plecanatide, while 1967 received placebo.
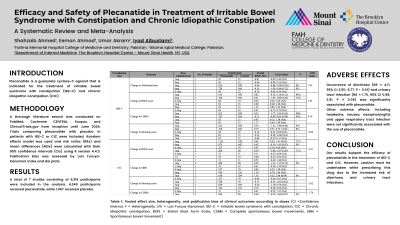
Decrease in abdominal pain in patients with IBS-C was significantly associated with the use of plecanatide (MD = -0.44, 95% CI -0.60; -0.27, P < 0.01).
Change in scores on the Bristol Stool Form Scale (BSFS) was significantly associated with the use of plecanatide in patients with IBS-C (MD = 0.78, 95% CI 0.47; 1.09, P < 0.01) and in patients with CIC (MD = 0.61, 95% CI 0.40; 0.83, P < 0.01).
Change in weekly complete spontaneous bowel movements was not significantly associated with either intervention or control for IBS-C (MD = 0.40, 95% CI -0.25; 1.06, P = 0.19). However, it was significantly associated with plecanatide for CIC (MD = 0.79, 95% CI 0.60; 0.98, P < 0.01), and so was change in weekly spontaneous bowel movements (MD = 1.11, 95% CI 0.77; 1.44, P < 0.01).
Change in straining score was not significant for the pooled data for IBS-C (MD = 0.26, 95% CI -0.18; 0.70, P = 0.20), although it significantly favoured placebo in the 6 mg subgroup (MD = 0.72, 95% CI 0.71; 0.73, P < 0.01). It was significantly associated with the use of plecanatide in patients with CIC (MD = -0.15, 95% CI -0.24; -0.05, P = 0.01).
Occurrence of diarrhoea (RR = 4.11, 95% CI 2.50; 6.77, P < 0.01) and urinary tract infection (RR = 1.70, 95% CI 0.99; 2.91, P = 0.05) was significantly associated with plecanatide, but none of the other adverse effects, including headache, nausea, nasopharyngitis and upper respiratory tract infection exhibited statistical significance.
Discussion: Our results support the efficacy of plecanatide in the treatment of IBS-C and CIC. However, caution must be undertaken while prescribing this drug due to the increased risk of diarrhoea and urinary tract infections.
Note: The table for this abstract can be viewed in the ePoster Gallery section of the ACG 2024 ePoster Site or in The American Journal of Gastroenterology's abstract supplement issue, both of which will be available starting October 27, 2024.
Disclosures:
Shahzaib Ahmed, MBBS1, Eeman Ahmad, MBBS1, Umar Akram, 2, Iyad Al-bustami, MD, MPH(c)3. P0627 - Efficacy and Safety of Plecanatide in Treatment of Irritable Bowel Syndrome With Constipation and Chronic Idiopathic Constipation, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
1Fatima Memorial Hospital College of Medicine and Dentistry, Lahore, Punjab, Pakistan; 2Allama Iqbal Medical College, Lahore, Punjab, Pakistan; 3Brooklyn Hospital Center, Houston, TX
Introduction: Plecanatide is a guanylate cyclase-C agonist that is indicated for the treatment of irritable bowel syndrome with constipation (IBS-C) and chronic idiopathic constipation (CIC).
Methods: We searched through PubMed, Cochrane CENTRAL, Scopus, and ClinicalTrials.gov from inception until June 2024. Trials comparing plecanatide with placebo in patients with IBS-C or CIC were included. Random effects model was used and risk ratios (RRs) and mean differences (MDs) were calculated with their 95% confidence intervals (CIs) using R version 4.4.0. Publication bias was assessed by Luis Furuya-Kanamori index and doi plots.
Results: A total of 7 studies consisting of 6316 participants were included in the analysis. 4349 participants received plecanatide, while 1967 received placebo.
Decrease in abdominal pain in patients with IBS-C was significantly associated with the use of plecanatide (MD = -0.44, 95% CI -0.60; -0.27, P < 0.01).
Change in scores on the Bristol Stool Form Scale (BSFS) was significantly associated with the use of plecanatide in patients with IBS-C (MD = 0.78, 95% CI 0.47; 1.09, P < 0.01) and in patients with CIC (MD = 0.61, 95% CI 0.40; 0.83, P < 0.01).
Change in weekly complete spontaneous bowel movements was not significantly associated with either intervention or control for IBS-C (MD = 0.40, 95% CI -0.25; 1.06, P = 0.19). However, it was significantly associated with plecanatide for CIC (MD = 0.79, 95% CI 0.60; 0.98, P < 0.01), and so was change in weekly spontaneous bowel movements (MD = 1.11, 95% CI 0.77; 1.44, P < 0.01).
Change in straining score was not significant for the pooled data for IBS-C (MD = 0.26, 95% CI -0.18; 0.70, P = 0.20), although it significantly favoured placebo in the 6 mg subgroup (MD = 0.72, 95% CI 0.71; 0.73, P < 0.01). It was significantly associated with the use of plecanatide in patients with CIC (MD = -0.15, 95% CI -0.24; -0.05, P = 0.01).
Occurrence of diarrhoea (RR = 4.11, 95% CI 2.50; 6.77, P < 0.01) and urinary tract infection (RR = 1.70, 95% CI 0.99; 2.91, P = 0.05) was significantly associated with plecanatide, but none of the other adverse effects, including headache, nausea, nasopharyngitis and upper respiratory tract infection exhibited statistical significance.
Discussion: Our results support the efficacy of plecanatide in the treatment of IBS-C and CIC. However, caution must be undertaken while prescribing this drug due to the increased risk of diarrhoea and urinary tract infections.
Note: The table for this abstract can be viewed in the ePoster Gallery section of the ACG 2024 ePoster Site or in The American Journal of Gastroenterology's abstract supplement issue, both of which will be available starting October 27, 2024.
Disclosures:
Shahzaib Ahmed indicated no relevant financial relationships.
Eeman Ahmad indicated no relevant financial relationships.
Umar Akram indicated no relevant financial relationships.
Iyad Al-bustami indicated no relevant financial relationships.
Shahzaib Ahmed, MBBS1, Eeman Ahmad, MBBS1, Umar Akram, 2, Iyad Al-bustami, MD, MPH(c)3. P0627 - Efficacy and Safety of Plecanatide in Treatment of Irritable Bowel Syndrome With Constipation and Chronic Idiopathic Constipation, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
